
In phosgene, $C-O$ bond length is longer than expected but $C-Cl$ bond length is shorter than expected. This is due to _________.
(A) Inductive effect
(B) Resonance effect
(C) Electronegativity effect
(D) None of these
Answer
583.2k+ views
Hint: The Inductive effect is a sigma electron transmission effect and has nothing to do with the double bond character and even the electronegativity effect is just the measure of the force of attraction by the nucleus. The Resonance involves the conversion of single and double bonds.
Complete step by step solution:
For this, we need to understand what these effects are and even what is the structure of phosgene. The type of bonds phosgene molecule has.
The first effect given is the inductive effect. It is the rise of the dipole in sigma bond due to unequal sharing of bonding electrons in the bond.
The second effect given is the resonance effect. The resonance effect is the induction of polarity in a bond due to lone pairs and a double bond.
The third effect is the electronegativity effect. It is the tendency of the nucleus to attract the valence electrons.
Now, let us see the structure of phosgene.
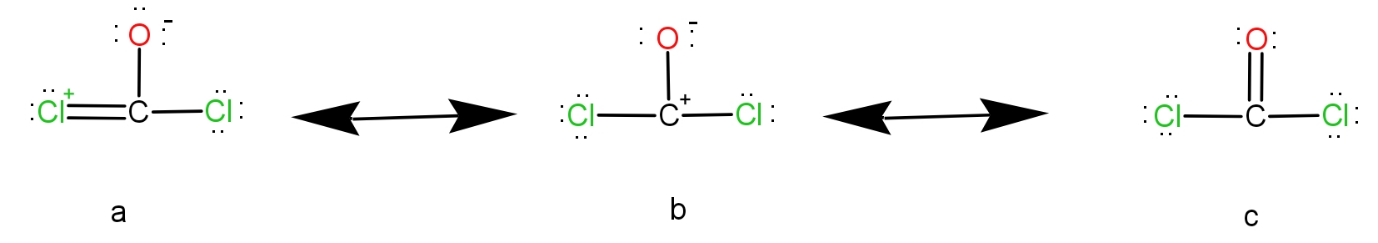
Observing the structures, we see that resonance is taking place between the double bond and lone pairs.
In actuality, the $C=O$ should be there and thus, it should be shorter while the $C-Cl$ bond should be single and thus should have a longer bond length. But due to resonance, the $C$ and $O$ bond acquires half double-bond character and half single bond character. This is the case with $C$ and $Cl$ bonds. So, the $C-O$ bond length increases and the $C-Cl$ bond length decreases.
Thus, option (B) is the correct answer.
Note: It must be noted that the electronegative effect is a measure of the force of attraction by the nucleus. It does not affect the bond length. Even the inductive effect does not have any effect on the bond length of the bond while in case of resonance because there is shifting of electrons resulting in the conversion of single bonds to double and vice versa. So, the bond length gets affected.
Complete step by step solution:
For this, we need to understand what these effects are and even what is the structure of phosgene. The type of bonds phosgene molecule has.
The first effect given is the inductive effect. It is the rise of the dipole in sigma bond due to unequal sharing of bonding electrons in the bond.
The second effect given is the resonance effect. The resonance effect is the induction of polarity in a bond due to lone pairs and a double bond.
The third effect is the electronegativity effect. It is the tendency of the nucleus to attract the valence electrons.
Now, let us see the structure of phosgene.

Observing the structures, we see that resonance is taking place between the double bond and lone pairs.
In actuality, the $C=O$ should be there and thus, it should be shorter while the $C-Cl$ bond should be single and thus should have a longer bond length. But due to resonance, the $C$ and $O$ bond acquires half double-bond character and half single bond character. This is the case with $C$ and $Cl$ bonds. So, the $C-O$ bond length increases and the $C-Cl$ bond length decreases.
Thus, option (B) is the correct answer.
Note: It must be noted that the electronegative effect is a measure of the force of attraction by the nucleus. It does not affect the bond length. Even the inductive effect does not have any effect on the bond length of the bond while in case of resonance because there is shifting of electrons resulting in the conversion of single bonds to double and vice versa. So, the bond length gets affected.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




