
In order to convert aniline into chlorobenzene, the reagents needed are-
(A) $CuCl$
(B) $NaN{{O}_{2}}/HCl\text{ and }CuCl$
(C) $C{{l}_{2}}/CC{{l}_{4}}$
(D) $C{{l}_{2}}/AlC{{l}_{3}}$
Answer
597.3k+ views
Hint: This reaction involves the formation of a diazonium salt. The second step of the reaction is commonly known as Sandmeyer reaction. Sandmeyer reaction is a radical nucleophilic aromatic substitution.
Complete-step- by- step solution:
The conversion of aniline to chlorobenzene actually consists of 2 steps. The first step is the reaction of aniline with $NaN{{O}_{2}}/HCl\text{ }$. This results in the formation of a diazonium salt, benzene diazonium chloride. This reaction occurs in the temperature range of $0-{{5}^{O}}C$ .
The next step is treating the diazonium salt with CuCl. This process is commonly known as the Sandmeyer reaction. This reaction is an example of a radical nucleophilic aromatic substitution.
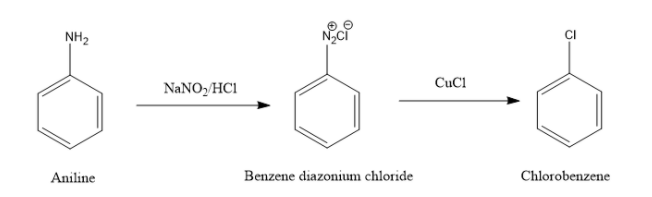
Option (A) cannot be correct as CuCl alone cannot cause this reaction. The CuCl is a reactant of the sandmeyer reaction. For sandmeyer reaction to happen, aniline first has to convert to benzene diazonium chloride.
Option (C) is incorrect. $C{{l}_{2}}/CC{{l}_{4}}$ is actually used as a chlorinating agent for alkenes. But it does not work for aniline.
Option (D) is also incorrect. Even though $C{{l}_{2}}/AlC{{l}_{3}}$ promotes chlorination of benzene. It does not work for aniline.
Thus, we can conclude that the answer is option (B) $NaN{{O}_{2}}/HCl\text{ and }CuCl$.
Additional Information:
There are various types of Sandmeyer reaction in which benzene diazonium chloride reacts with CuCl, CuBr, CuCN to form chlorobenzene, bromobenzene and benzonitrile respectively.
Note: Even though CuCl is the reagent used for sandmeyer reaction, option (A) is incorrect. This is because for sandmeyer reaction to happen, aniline first has to convert to benzene diazonium chloride.
Complete-step- by- step solution:
The conversion of aniline to chlorobenzene actually consists of 2 steps. The first step is the reaction of aniline with $NaN{{O}_{2}}/HCl\text{ }$. This results in the formation of a diazonium salt, benzene diazonium chloride. This reaction occurs in the temperature range of $0-{{5}^{O}}C$ .
The next step is treating the diazonium salt with CuCl. This process is commonly known as the Sandmeyer reaction. This reaction is an example of a radical nucleophilic aromatic substitution.

Option (A) cannot be correct as CuCl alone cannot cause this reaction. The CuCl is a reactant of the sandmeyer reaction. For sandmeyer reaction to happen, aniline first has to convert to benzene diazonium chloride.
Option (C) is incorrect. $C{{l}_{2}}/CC{{l}_{4}}$ is actually used as a chlorinating agent for alkenes. But it does not work for aniline.
Option (D) is also incorrect. Even though $C{{l}_{2}}/AlC{{l}_{3}}$ promotes chlorination of benzene. It does not work for aniline.
Thus, we can conclude that the answer is option (B) $NaN{{O}_{2}}/HCl\text{ and }CuCl$.
Additional Information:
There are various types of Sandmeyer reaction in which benzene diazonium chloride reacts with CuCl, CuBr, CuCN to form chlorobenzene, bromobenzene and benzonitrile respectively.
Note: Even though CuCl is the reagent used for sandmeyer reaction, option (A) is incorrect. This is because for sandmeyer reaction to happen, aniline first has to convert to benzene diazonium chloride.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




