
Square planar complexes of the type MAXBL (where A,B,X,L are unidentate ligands) show the following set of isomers.
a.) Two cis and one trans
b.) Two trans and one cis
c.) Two cis and two trans
d.) Three cis and one trans
Answer
595.5k+ views
Hint: A square planar complex having similar ligands at adjacent positions (${90^0}$) is called cis isomer while the complex with similar ligands at opposite positions (${180^0}$) is called trans isomer.
Complete step by step answer:
The complex compounds which have the same ligand in the coordination sphere but relative positions of ligands round the central metal atom are different are called geometrical isomers and the phenomenon is called geometrical isomerism. The square planar complexes with coordination number four shows cis-trans geometrical isomerism.
Unidentate ligands are those which binds through only one site.
Normally when we see cis-trans isomerism, we have two ligands same on basis of which we differentiate in cis and trans. As all four ligands are different here, we will first assume that ‘A’ and ‘B’ are similar while ‘X’ and ‘L’ are similar. Thus, on the basis of this we will draw the structure as-
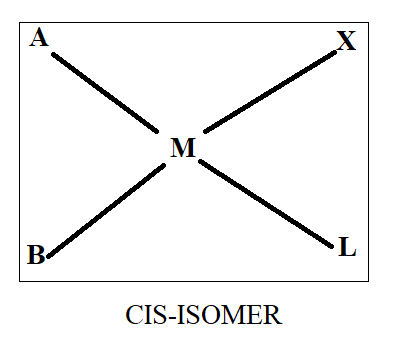
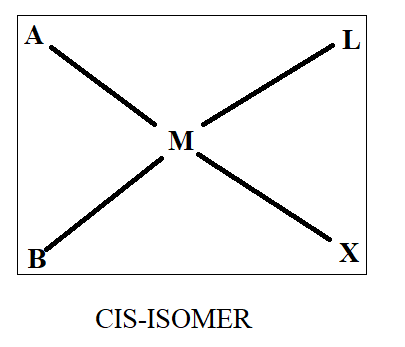
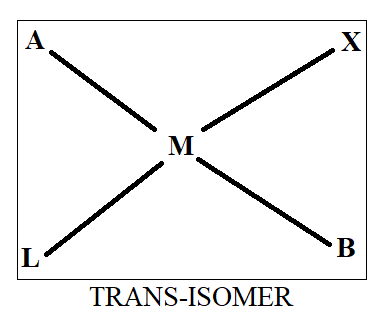
Thus, we get three structures of which two are in cis form while one is in trans form.
Example- $\left[ {P{t^{2 + }}\left( {N{H_3}} \right)\left( {py} \right)\left( {Cl} \right)\left( {Br} \right)} \right]$ So, the correct answer is “Option A”.
Note: Geometrical isomerism can-not be shown by tetrahedral complexes because all four ligands are in adjacent positions and all the four bond angles are the same.
Complete step by step answer:
The complex compounds which have the same ligand in the coordination sphere but relative positions of ligands round the central metal atom are different are called geometrical isomers and the phenomenon is called geometrical isomerism. The square planar complexes with coordination number four shows cis-trans geometrical isomerism.
Unidentate ligands are those which binds through only one site.
Normally when we see cis-trans isomerism, we have two ligands same on basis of which we differentiate in cis and trans. As all four ligands are different here, we will first assume that ‘A’ and ‘B’ are similar while ‘X’ and ‘L’ are similar. Thus, on the basis of this we will draw the structure as-



Thus, we get three structures of which two are in cis form while one is in trans form.
Example- $\left[ {P{t^{2 + }}\left( {N{H_3}} \right)\left( {py} \right)\left( {Cl} \right)\left( {Br} \right)} \right]$ So, the correct answer is “Option A”.
Note: Geometrical isomerism can-not be shown by tetrahedral complexes because all four ligands are in adjacent positions and all the four bond angles are the same.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




