
In \[N_3^ - \] ion, the number of bond pair and lone pair of electron on nitrogen atom are:
A. 2,2
B. 3,1
C. 1,3
D. 4,0
Answer
565.5k+ views
Hint: Lone pair is the pair of valence electrons that are not involved in bonding whereas bond pair is the pair of valence electrons involved in bonding. The nitrogen atom has five valence electrons, so it can accept three electrons to attain an octet configuration. Thus, the valency of nitrogen is +3 and it has one lone pair of electrons.
Complete step by step answer:
Generally, the Lone pair is the pair of valence electrons that are not involved in bonding whereas the bond pair is the pair of valence electrons involved in bonding.
For example, in a nitrogen atom, its outermost electronic configuration can be written as,
N-atom is \[2{s^2}2{p^3}\]
We all are known that the valency of nitrogen is 3. Thus, it has three electrons for bonding with other elements and it has one lone pair for non-bonding.
The structure of \[N_3^ - \] ion can be drawn as,
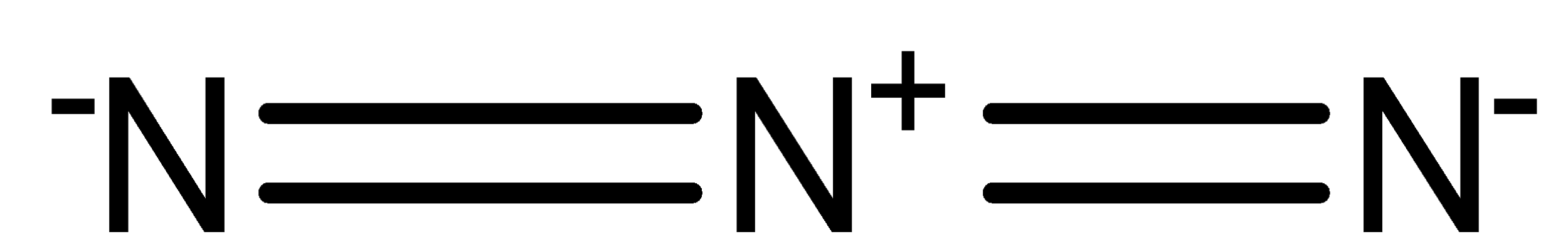
Generally, the positive sign refers that the electron is insufficient for that element and the negative sign refers that the electron is excess for that compound.
In \[N_3^ - \] structure, the terminal nitrogen on both sides has one excess lone pair of electrons and thus, forming a negative charge. Whereas in between nitrogen atoms lost its lone pair via bonding and thus, forming positive charge.
From the above structure of \[N_3^ - \] ion structure, the nitrogen atom present in the center of \[N_3^ - \] ion has 0 lone pair of electrons and 4 bond pairs of electrons.
Thus, In \[N_3^ - \] ion, the number of lone pair and bond pair of electron on the nitrogen atom is an option (D).
Note: Nitrogen atom present in the center of \[N_3^ - \] ion has 0 lone pair of electrons and 4 bond pairs of electrons. While the terminal nitrogen atom of \[N_3^ - \] ion has two lone pairs and two bond pairs of electrons. Nitride compounds are mainly used as insulators. Silicon nitride and titanium nitride are used as cutting materials and hard coatings.
Complete step by step answer:
Generally, the Lone pair is the pair of valence electrons that are not involved in bonding whereas the bond pair is the pair of valence electrons involved in bonding.
For example, in a nitrogen atom, its outermost electronic configuration can be written as,
N-atom is \[2{s^2}2{p^3}\]
We all are known that the valency of nitrogen is 3. Thus, it has three electrons for bonding with other elements and it has one lone pair for non-bonding.
The structure of \[N_3^ - \] ion can be drawn as,
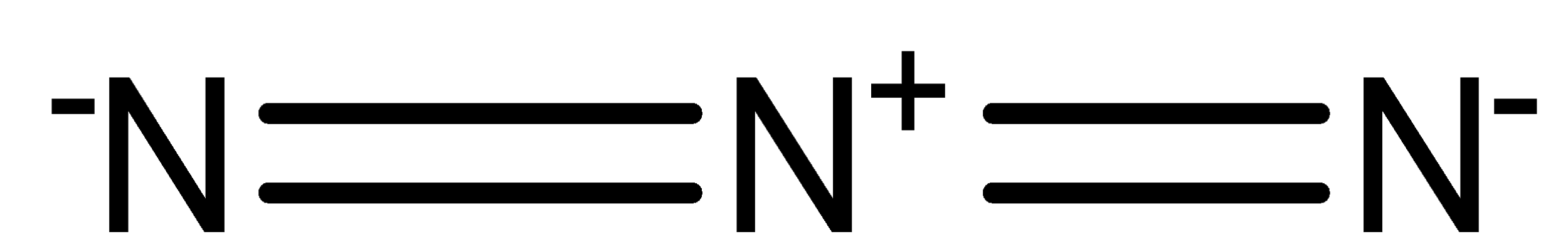
Generally, the positive sign refers that the electron is insufficient for that element and the negative sign refers that the electron is excess for that compound.
In \[N_3^ - \] structure, the terminal nitrogen on both sides has one excess lone pair of electrons and thus, forming a negative charge. Whereas in between nitrogen atoms lost its lone pair via bonding and thus, forming positive charge.
From the above structure of \[N_3^ - \] ion structure, the nitrogen atom present in the center of \[N_3^ - \] ion has 0 lone pair of electrons and 4 bond pairs of electrons.
Thus, In \[N_3^ - \] ion, the number of lone pair and bond pair of electron on the nitrogen atom is an option (D).
Note: Nitrogen atom present in the center of \[N_3^ - \] ion has 0 lone pair of electrons and 4 bond pairs of electrons. While the terminal nitrogen atom of \[N_3^ - \] ion has two lone pairs and two bond pairs of electrons. Nitride compounds are mainly used as insulators. Silicon nitride and titanium nitride are used as cutting materials and hard coatings.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




