
In lead acid battery electrolyte used is:
A.Sulphuric acid
B.Aqueous solution of $PbS{O_4}$
C.Nitric acid
D.Aqueous solution of $FeS{O_4}$
Answer
571.5k+ views
Hint: As we know that the lead acid battery will have two electrodes and an electrolytic solution. The electrodes present in lead acid batteries are lead and lead dioxide.
Complete step by step answer:Lead acid batteries have a small energy-to-volume ratio and a very low energy-to-weight ratio; its ability to supply high contents reveals that the cell has a large power-to-weight ratio. Lead-acid batteries are categorized by secondary batteries. The chemical reactions, which occur in secondary cells, are reversible.
The commercial forms of lead acid batteries consist of six (or) twelve lead storage cells linked together.
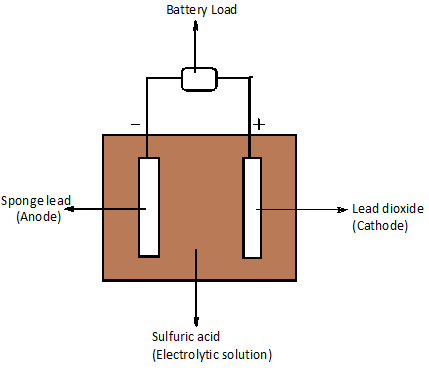
Lead storage batteries are otherwise called lead-acid batteries. It is a secondary voltaic cell. The anode is made up of spongy porous lead and the cathode is made up of lead dioxide. The electrolyte used is sulfuric acid.
The active materials present on the plate of the battery (electrodes) are lead and lead dioxide. They react with sulfuric acid (electrolyte) to form lead sulfate. The formed lead sulfate is in amorphous state, finely divided and when the battery recharges it again forms lead, lead dioxide and sulfuric acid.
Lead acid batteries are used in large backup power supplies for telephone and compound centers, grid energy storage and as emergency lighting and to power sump pumps in power failure.
Therefore, the option (A) is correct.
Note:As we that lead storage batteries are cost efficient. They have low internal impedance and are tolerant to overcharging. They are also heavy and bulky with a cycle life of 300 to 500 cycles. There are varieties of lead acid batteries such as lead calcium battery, lead antimony battery etc.
Complete step by step answer:Lead acid batteries have a small energy-to-volume ratio and a very low energy-to-weight ratio; its ability to supply high contents reveals that the cell has a large power-to-weight ratio. Lead-acid batteries are categorized by secondary batteries. The chemical reactions, which occur in secondary cells, are reversible.
The commercial forms of lead acid batteries consist of six (or) twelve lead storage cells linked together.
Lead storage batteries are otherwise called lead-acid batteries. It is a secondary voltaic cell. The anode is made up of spongy porous lead and the cathode is made up of lead dioxide. The electrolyte used is sulfuric acid.
The active materials present on the plate of the battery (electrodes) are lead and lead dioxide. They react with sulfuric acid (electrolyte) to form lead sulfate. The formed lead sulfate is in amorphous state, finely divided and when the battery recharges it again forms lead, lead dioxide and sulfuric acid.

Lead acid batteries are used in large backup power supplies for telephone and compound centers, grid energy storage and as emergency lighting and to power sump pumps in power failure.
Therefore, the option (A) is correct.
Note:As we that lead storage batteries are cost efficient. They have low internal impedance and are tolerant to overcharging. They are also heavy and bulky with a cycle life of 300 to 500 cycles. There are varieties of lead acid batteries such as lead calcium battery, lead antimony battery etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




