
In ammonia molecule polar covalent bond formation takes place between nitrogen and hydrogen.
A. True
B. False
Answer
556.5k+ views
Hint: We need to remember that the non-metals have shells of electrons that are normally half or quite half filled with electrons. Since they need a robust attraction for a couple of additional electrons. But this is energetically unfavorable for any atom to lose electrons, in order that they share electrons by overlapping orbitals. This is a covalent bond and this consists of two or more electrons.
Complete answer:
We must know that the ammonia chemical formula is $N{H_3}$. It constitutes nitrogen $(N)$ and hydrogen $(H)$, it is a pungent gas. It’s the stable compound for those elements and is a starting material for the manufacture of the many commercially vital nitrogen compounds.
As we know that the atomic number of nitrogen and hydrogen is $7$ and $1$ respectively. Outermost electronic configuration of nitrogen is $2{s^2}2{p^3}$ and hydrogen electronic configuration is $1{s^1}$.
Nitrogen, if it completes the octet or noble gas configuration, then it gains or accepts three electrons. Here, ammonia forming, not to be ionic, is a covalent bond. In covalent bonds, electrons are represented by dots.
Electron dot structure of nitrogen and hydrogen is respectively.
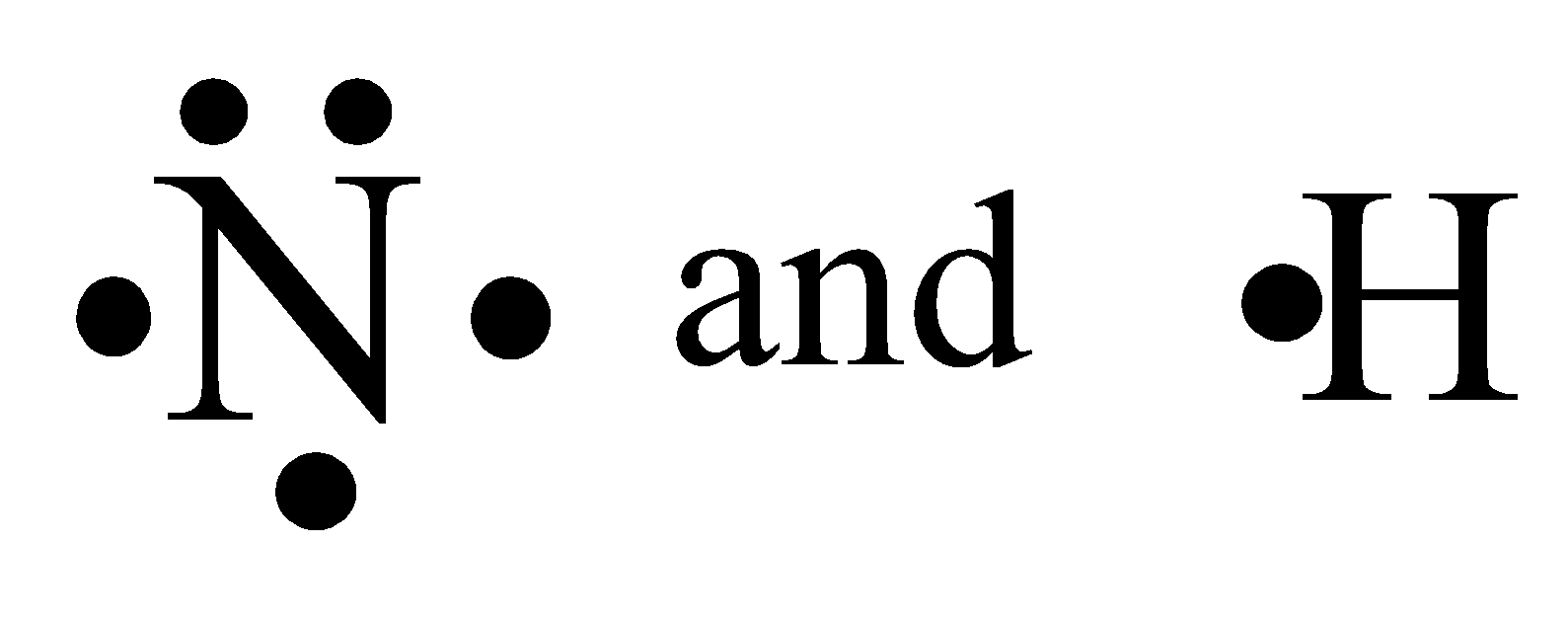
We must remember that the hydrogen can be given this one number of electrons to the nitrogen and then its existence will not remain so they will do the sharing of electrons and will make the bond.
Hydrogen will not donate an electron to the nitrogen or nitrogen will not take the hydrogen but they will share the electron with each other.
In nitrogen, three electrons to complete it octet. One electron from the hydrogen is not sufficient for this nitrogen, so the nitrogen will make bond with the three different hydrogen.
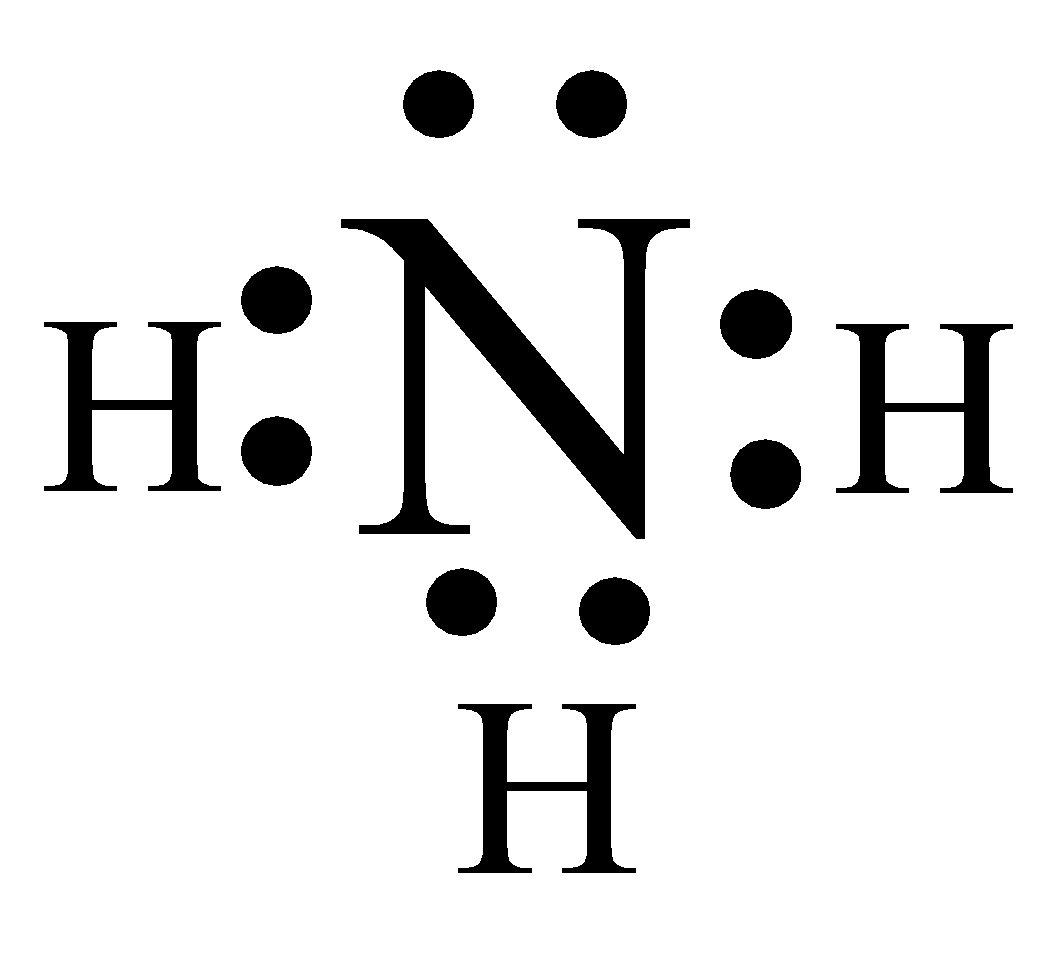
In the above structure, above the nitrogen is an electron pair and is not involved in bond formation.
Polar molecule: A polar molecule features a net dipole as a result of the opposing charges from the polar bonds, which are arranged asymmetrically.
We can draw the structure of ammonia as,
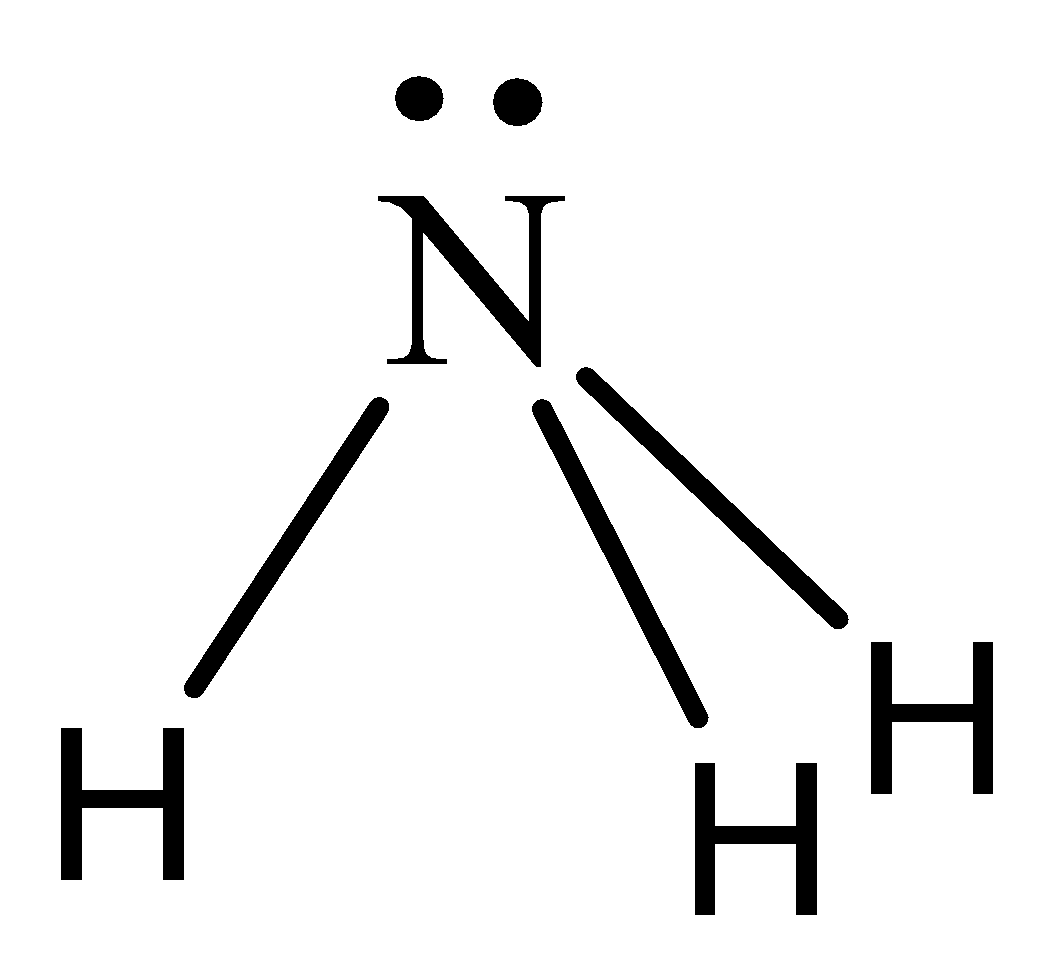
We need to remember that the ammonia $(N{H_3})$ molecule has trigonal pyramidal structure and it is a polar in nature due to its asymmetrical shape and also different in electronegativities of nitrogen $(3.04)$ and hydrogen $(2.2)$. The nitrogen and hydrogen charges are unequally distributed which ends up during a net moment making ammonia is a polar molecule.
Therefore, in ammonia molecules polar covalent bond formation takes place between nitrogen and hydrogen.
Therefore, The given statement is (A) “True”.
Note:
As we know that the bonding between atoms of a molecule and therefore the lone pairs of electrons which will exist within the molecule is shown by Lewis dot structures (LEDS). Gilbert N. Lewis introduced the idea of covalent bond. Lewis structures widen the concept of the electron dot illustration by adding lines between atoms to show shared pairs in a chemical bond.
Complete answer:
We must know that the ammonia chemical formula is $N{H_3}$. It constitutes nitrogen $(N)$ and hydrogen $(H)$, it is a pungent gas. It’s the stable compound for those elements and is a starting material for the manufacture of the many commercially vital nitrogen compounds.
As we know that the atomic number of nitrogen and hydrogen is $7$ and $1$ respectively. Outermost electronic configuration of nitrogen is $2{s^2}2{p^3}$ and hydrogen electronic configuration is $1{s^1}$.
Nitrogen, if it completes the octet or noble gas configuration, then it gains or accepts three electrons. Here, ammonia forming, not to be ionic, is a covalent bond. In covalent bonds, electrons are represented by dots.
Electron dot structure of nitrogen and hydrogen is respectively.
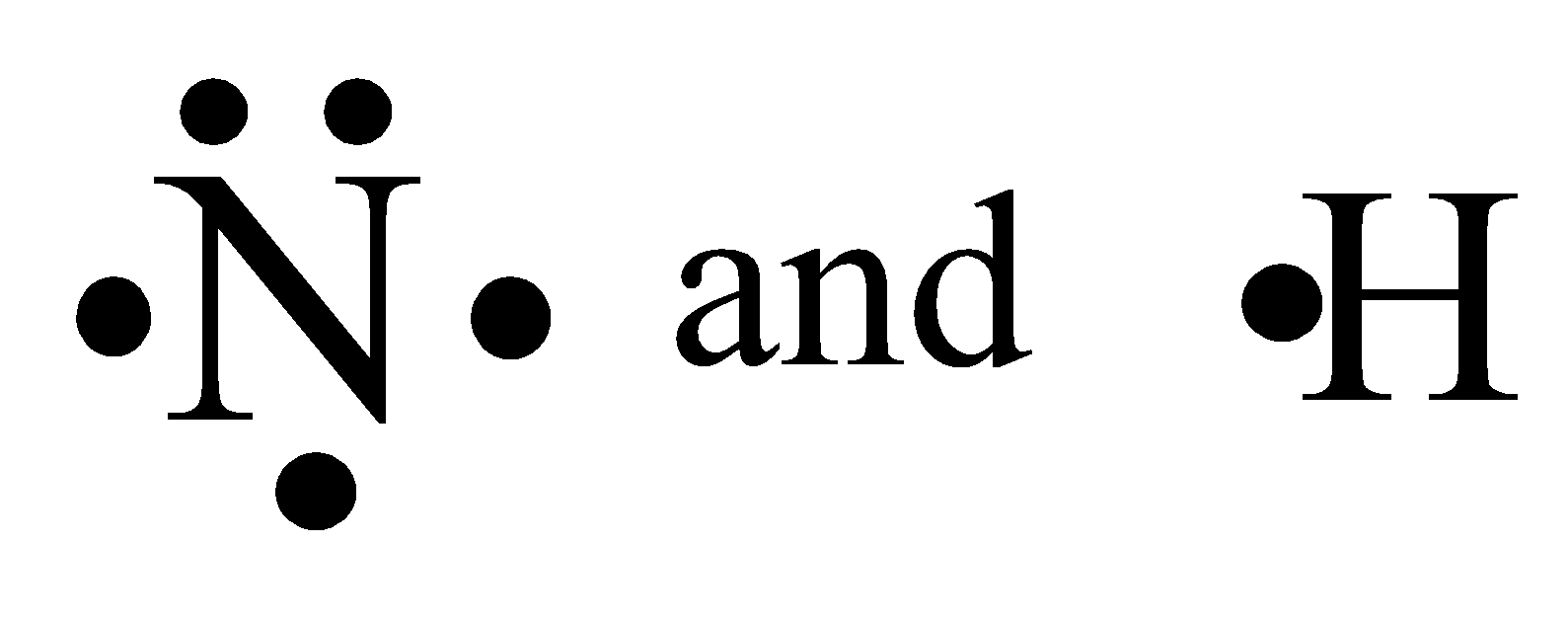
We must remember that the hydrogen can be given this one number of electrons to the nitrogen and then its existence will not remain so they will do the sharing of electrons and will make the bond.
Hydrogen will not donate an electron to the nitrogen or nitrogen will not take the hydrogen but they will share the electron with each other.
In nitrogen, three electrons to complete it octet. One electron from the hydrogen is not sufficient for this nitrogen, so the nitrogen will make bond with the three different hydrogen.

In the above structure, above the nitrogen is an electron pair and is not involved in bond formation.
Polar molecule: A polar molecule features a net dipole as a result of the opposing charges from the polar bonds, which are arranged asymmetrically.
We can draw the structure of ammonia as,

We need to remember that the ammonia $(N{H_3})$ molecule has trigonal pyramidal structure and it is a polar in nature due to its asymmetrical shape and also different in electronegativities of nitrogen $(3.04)$ and hydrogen $(2.2)$. The nitrogen and hydrogen charges are unequally distributed which ends up during a net moment making ammonia is a polar molecule.
Therefore, in ammonia molecules polar covalent bond formation takes place between nitrogen and hydrogen.
Therefore, The given statement is (A) “True”.
Note:
As we know that the bonding between atoms of a molecule and therefore the lone pairs of electrons which will exist within the molecule is shown by Lewis dot structures (LEDS). Gilbert N. Lewis introduced the idea of covalent bond. Lewis structures widen the concept of the electron dot illustration by adding lines between atoms to show shared pairs in a chemical bond.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




