
In a double bond between two carbon atoms of ethane, there are
A. Two sigma bonds perpendicular to each other
B. One pi bond and one sigma bond
C. Two pi bonds perpendicular to each other
D. Two pi bonds at an angle of \[60{}^\circ \]
Answer
363.9k+ views
Hint: As the bond between two carbons is double and other bond are single (ethene) this means that the hybridization of both carbon atoms is \[s{{p}^{2}}\]. The hybridization \[s{{p}^{2}}\] means that there are three orbital’s of the same energy (\[s{{p}^{2}}\]) but carbon can form four bonds as per its valence but due to the lack of any other atom (two hydrogen and one bond between both carbons) fourth bond formed between the two carbon atom again (pz orbital of both carbons overlap sidewise).
Complete Step by Step Answer:
The carbon atom whose atomic number is 6 and in its first exited state its electronic configuration is given as \[2{{s}^{1}},\text{ }2{{p}^{3}}\]. So, carbon has a tendency to form four single bonds with different atoms. The energy of s and p orbital of second shell has somewhere the same energy thus, due to hybridization rules all the resultant orbital have the same energy such as all the orbital overlap such as 1 + 3 (one orbital of s and 3 orbital of p) forming 4 orbitals of same energy \[s{{p}^{3}}\]such as
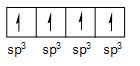
But in ethene one carbon (and also other) is bonded with two hydrogen atom with single both (sigma bond) and one carbon atom also with single bond (sigma bond) means only \[2{{s}^{1}}\] orbital and \[2{{p}^{2}}\]orbital of carbon is overlapping giving out resultant orbital of same energy (1 of s + 2 of p = 3 of \[s{{p}^{2}}\]energy) as per hybridization rule. And the last pz orbital of both carbon form bond with each other in such a way that they form pi bond (pz orbital of both carbon join together with sidewise linking) such as
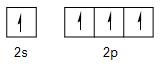
Thus, the correct option is B as there is three sigma bond and one pi bond in each carbon of ethene or can say one sigma bond and one pi bond between both the carbons.
Note: It is important to note that the s orbital due to its symmetry shape has no orientation in space due to which it cannot form with other s or any other orbital through side wise linkage due to which it cannot form pi bond whereas the p orbital has three orientation in space x, y, and z due to which it can form bond with other p or any other orbital accept orbital with side wise linkage and thus can form pi bond. In ethene there are four single bonds and one double bond (double bond is always formed by one single bond and one pi bond).
Complete Step by Step Answer:
The carbon atom whose atomic number is 6 and in its first exited state its electronic configuration is given as \[2{{s}^{1}},\text{ }2{{p}^{3}}\]. So, carbon has a tendency to form four single bonds with different atoms. The energy of s and p orbital of second shell has somewhere the same energy thus, due to hybridization rules all the resultant orbital have the same energy such as all the orbital overlap such as 1 + 3 (one orbital of s and 3 orbital of p) forming 4 orbitals of same energy \[s{{p}^{3}}\]such as

But in ethene one carbon (and also other) is bonded with two hydrogen atom with single both (sigma bond) and one carbon atom also with single bond (sigma bond) means only \[2{{s}^{1}}\] orbital and \[2{{p}^{2}}\]orbital of carbon is overlapping giving out resultant orbital of same energy (1 of s + 2 of p = 3 of \[s{{p}^{2}}\]energy) as per hybridization rule. And the last pz orbital of both carbon form bond with each other in such a way that they form pi bond (pz orbital of both carbon join together with sidewise linking) such as

Thus, the correct option is B as there is three sigma bond and one pi bond in each carbon of ethene or can say one sigma bond and one pi bond between both the carbons.
Note: It is important to note that the s orbital due to its symmetry shape has no orientation in space due to which it cannot form with other s or any other orbital through side wise linkage due to which it cannot form pi bond whereas the p orbital has three orientation in space x, y, and z due to which it can form bond with other p or any other orbital accept orbital with side wise linkage and thus can form pi bond. In ethene there are four single bonds and one double bond (double bond is always formed by one single bond and one pi bond).
Recently Updated Pages
Master Class 8 Social Science: Engaging Questions & Answers for Success

Master Class 8 English: Engaging Questions & Answers for Success

Class 8 Question and Answer - Your Ultimate Solutions Guide

Master Class 8 Maths: Engaging Questions & Answers for Success

Master Class 8 Science: Engaging Questions & Answers for Success

Master Class 7 English: Engaging Questions & Answers for Success

Trending doubts
What are the factors of 100 class 7 maths CBSE

Which are the Top 10 Largest Countries of the World?

What is BLO What is the full form of BLO class 8 social science CBSE

The value of 6 more than 7 is A 1 B 1 C 13 D 13 class 7 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE




