
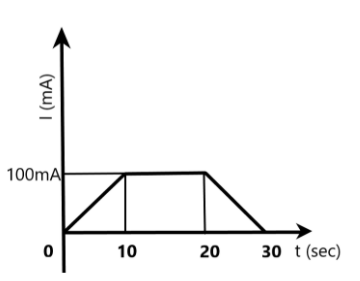
In a copper voltameter, the mass deposited in the 30s is m gram. If the current time graph is as shown in the figure, the electrochemical equivalent of copper, in gm/coulomb, will be –
\[\begin{align}
& \text{A) m} \\
& \text{B) }\dfrac{\text{m}}{\text{2}} \\
& \text{C) 0}\text{.06m} \\
& \text{D) 0}\text{.1m} \\
\end{align}\]

Answer
573.6k+ views
Hint: We need to find the electrochemical equivalent of the given metal whose mass deposited and time of electrolysis is given. We can use the information on the mass of the metal deposited in the given time to find the required electro chemical equivalent.
Complete answer:
The voltameter is a device which is used in the electrolytic reactions to find the amount of electric charge deposited or carried away with the utilization of a unit of charge. In the situation given to us, such a voltmeter using the copper metal is used.
The copper voltameter when functioned for 30s deposits ‘m’ grams of the copper on the electrode. We need to find the electrochemical equivalent of the metal copper using this information.
The electrochemical equivalent of the electrode is the measure of the mass transferred from one electrode in an electrolytic cell to the other by the application of a unit amount of charge, 1 coulomb.
In this case, we are given the current applied to the cell for 30s from the graph. The area under the graph, as we can see clearly-a trapezium, gives the product of the current and time, i.e., the total charge involved in the cell for the 30s.
We can find the area of the trapezium as –
\[\begin{align}
& \text{Area of trapezium, A}=2\times \dfrac{1}{2}bh+{{a}^{2}} \\
& \Rightarrow A=(10s\times 100mA)+(10s\times 100mA) \\
& \Rightarrow A=2000mC=2C \\
\end{align}\]
The total charge is given as the area under the graph, which is 2 coulombs.
Now, we can find the electrochemical equivalent of the copper metal as the ratio between the mass and the charge involved as –
\[\begin{align}
& Z=\dfrac{m}{Q} \\
& \therefore Z=\dfrac{m}{2}gm{{C}^{-1}} \\
\end{align}\]
The electrochemical equivalent of copper is \[Z=\dfrac{m}{2}gm{{C}^{-1}}\].
The correct answer is option B.
Note:
The voltameter and the voltmeter are two different electrical devices. The voltmeter is the device which measures the potential drop in a circuit, whereas the voltmeter measures the amount of electric current that is involved in an electrolytic cell.
Complete answer:
The voltameter is a device which is used in the electrolytic reactions to find the amount of electric charge deposited or carried away with the utilization of a unit of charge. In the situation given to us, such a voltmeter using the copper metal is used.
The copper voltameter when functioned for 30s deposits ‘m’ grams of the copper on the electrode. We need to find the electrochemical equivalent of the metal copper using this information.
The electrochemical equivalent of the electrode is the measure of the mass transferred from one electrode in an electrolytic cell to the other by the application of a unit amount of charge, 1 coulomb.
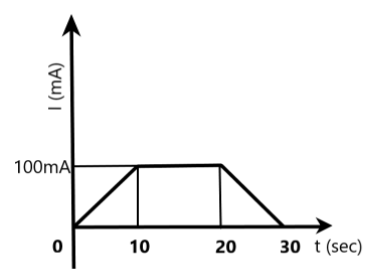
In this case, we are given the current applied to the cell for 30s from the graph. The area under the graph, as we can see clearly-a trapezium, gives the product of the current and time, i.e., the total charge involved in the cell for the 30s.

We can find the area of the trapezium as –
\[\begin{align}
& \text{Area of trapezium, A}=2\times \dfrac{1}{2}bh+{{a}^{2}} \\
& \Rightarrow A=(10s\times 100mA)+(10s\times 100mA) \\
& \Rightarrow A=2000mC=2C \\
\end{align}\]
The total charge is given as the area under the graph, which is 2 coulombs.
Now, we can find the electrochemical equivalent of the copper metal as the ratio between the mass and the charge involved as –
\[\begin{align}
& Z=\dfrac{m}{Q} \\
& \therefore Z=\dfrac{m}{2}gm{{C}^{-1}} \\
\end{align}\]
The electrochemical equivalent of copper is \[Z=\dfrac{m}{2}gm{{C}^{-1}}\].
The correct answer is option B.
Note:
The voltameter and the voltmeter are two different electrical devices. The voltmeter is the device which measures the potential drop in a circuit, whereas the voltmeter measures the amount of electric current that is involved in an electrolytic cell.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




