
If $E_{_{A{u^ + }/Au}}^0 = 1.69V$and $E_{A{u^{ + 3}}/Au}^0 = 1.40V$then $E_{A{u^ + }/A{u^{3 + }}}^0$ will it be?
A.$0.19V$
B.$2.495V$
C.$ - 2.5V$
D.$ - 1.255V$
Answer
564.6k+ views
Hint:The electrode potential is the electromotive force of a galvanic cell which is calculated as the standard reference electrode and another electrode. We take the electrode potential of anode on the left and electrode potential of cathode on the right. This gives us the electromotive force of a cell.
Formula used:
${n_3}{E_3} = {n_2}{E_2} + {n_1}{E_1}$
Complete step by step answer:
The potential difference between the two electrodes of a galvanic cell is known as the cell potential which is calculated in volts and the cell potential is calculated as the difference between the electrode potentials of the cathode and anode. We take the electrode potential of anode on the left and electrode potential of cathode on the right. This gives us the electromotive force of a cell. Thus, electrode potential is the electromotive force of a galvanic cell which is calculated as the standard reference electrode and another electrode.
${E_{cell}} = {E_{cathode}} + {E_{anode}}$
The hydrogen electrode is taken as the standard electrode as it is meant to have a potential of zero volts. Oxidation occurs at the anode and reduction occurs at the cathode.
${H^ + }(aq) + {e^ - } = \dfrac{1}{2}{H_2}(g)$
For the above question, it is given that
$E_{_{A{u^ + }/Au}}^0 = 1.69V$
$ \Rightarrow A{u^ + } + 1{e^ - } \to Au$
$E_{A{u^{ + 3}}/Au}^0 = 1.40V$
$ \Rightarrow A{u^{3 + }} + 3{e^ - } \to Au$
${E_{A{u^ + }/A{u^{ + 3}}}} \Rightarrow A{u^ + } \to A{u^{ + 3}} + 2{e^ - }$
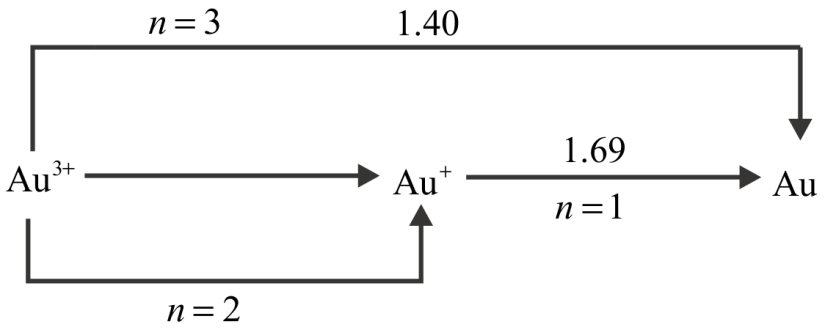
We know that,
${n_3}{E_3} = {n_2}{E_2} + {n_1}{E_1}$
$ \Rightarrow 3 \times 1.40 = 1 \times 1.69 + 2 \times E_{A{u^{3 + }}/A{u^ + }}^0$
${E^0}_{A{u^{3 + }}/A{u^ + }} = \dfrac{{4.20 - 1.69}}{2}$
$ \Rightarrow {E^0}_{A{u^{3 + }}/A{u^ + }} = 1.255$
We have to calculate $E_{A{u^ + }/A{u^{3 + }}}^0$
$ \Rightarrow E_{A{u^ + }/A{u^{3 + }}}^0 = - E_{A{u^{3 + }}/A{u^ + }}^0$
$ \Rightarrow E_{A{u^ + }/A{u^{3 + }}}^0 = - 1.255$
Hence, the correct option is (D) .
The reduced form of an electrode is more stable than the hydrogen electrode if its standard electrode potential is greater than zero.
Note:
Platinum and gold are metals which are used as electrodes and are known as inert electrodes because they do not react in the reaction and instead provide their surface for reactions like oxidation and reduction to take place and conduct electrons and complete the galvanic cell circuit.
Formula used:
${n_3}{E_3} = {n_2}{E_2} + {n_1}{E_1}$
Complete step by step answer:
The potential difference between the two electrodes of a galvanic cell is known as the cell potential which is calculated in volts and the cell potential is calculated as the difference between the electrode potentials of the cathode and anode. We take the electrode potential of anode on the left and electrode potential of cathode on the right. This gives us the electromotive force of a cell. Thus, electrode potential is the electromotive force of a galvanic cell which is calculated as the standard reference electrode and another electrode.
${E_{cell}} = {E_{cathode}} + {E_{anode}}$
The hydrogen electrode is taken as the standard electrode as it is meant to have a potential of zero volts. Oxidation occurs at the anode and reduction occurs at the cathode.
${H^ + }(aq) + {e^ - } = \dfrac{1}{2}{H_2}(g)$
For the above question, it is given that
$E_{_{A{u^ + }/Au}}^0 = 1.69V$
$ \Rightarrow A{u^ + } + 1{e^ - } \to Au$
$E_{A{u^{ + 3}}/Au}^0 = 1.40V$
$ \Rightarrow A{u^{3 + }} + 3{e^ - } \to Au$
${E_{A{u^ + }/A{u^{ + 3}}}} \Rightarrow A{u^ + } \to A{u^{ + 3}} + 2{e^ - }$

We know that,
${n_3}{E_3} = {n_2}{E_2} + {n_1}{E_1}$
$ \Rightarrow 3 \times 1.40 = 1 \times 1.69 + 2 \times E_{A{u^{3 + }}/A{u^ + }}^0$
${E^0}_{A{u^{3 + }}/A{u^ + }} = \dfrac{{4.20 - 1.69}}{2}$
$ \Rightarrow {E^0}_{A{u^{3 + }}/A{u^ + }} = 1.255$
We have to calculate $E_{A{u^ + }/A{u^{3 + }}}^0$
$ \Rightarrow E_{A{u^ + }/A{u^{3 + }}}^0 = - E_{A{u^{3 + }}/A{u^ + }}^0$
$ \Rightarrow E_{A{u^ + }/A{u^{3 + }}}^0 = - 1.255$
Hence, the correct option is (D) .
The reduced form of an electrode is more stable than the hydrogen electrode if its standard electrode potential is greater than zero.
Note:
Platinum and gold are metals which are used as electrodes and are known as inert electrodes because they do not react in the reaction and instead provide their surface for reactions like oxidation and reduction to take place and conduct electrons and complete the galvanic cell circuit.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




