
How do you identify sigma and pi-bonds?
Answer
565.2k+ views
Hint:. The answer to this question is based on the fact that these two bonds are covalent bonds which are formed by the overlapping of the atomic orbitals and thus based on this fact you can approach the required answer.
Complete step by step answer:
In the lower classes of chemistry, we have studied about the basic concepts of the general chemistry part which deals with the facts that include the concept of types of bonds formed between the atoms in a molecule such as ionic bid, covalent bond, coordinate bond etc.
Let us know what sigma and pi bonds are and how it can be identified.
- Sigma and pi – bonds are the two covalent bonds that are formed between the atoms in a molecule according to their valence.
- Since we know that a covalent bond is formed by sharing a single electron from each atom, the first bond formed will be the sigma bond which is formed by the head on overlap of s – s or s – p or p – p along the internuclear axis.
Similarly, pi bonds are formed by the head on overlapping of parallel p orbitals on adjacent atoms.
In an example of ethane molecule, the sigma and pi bonds formed are depicted as below,
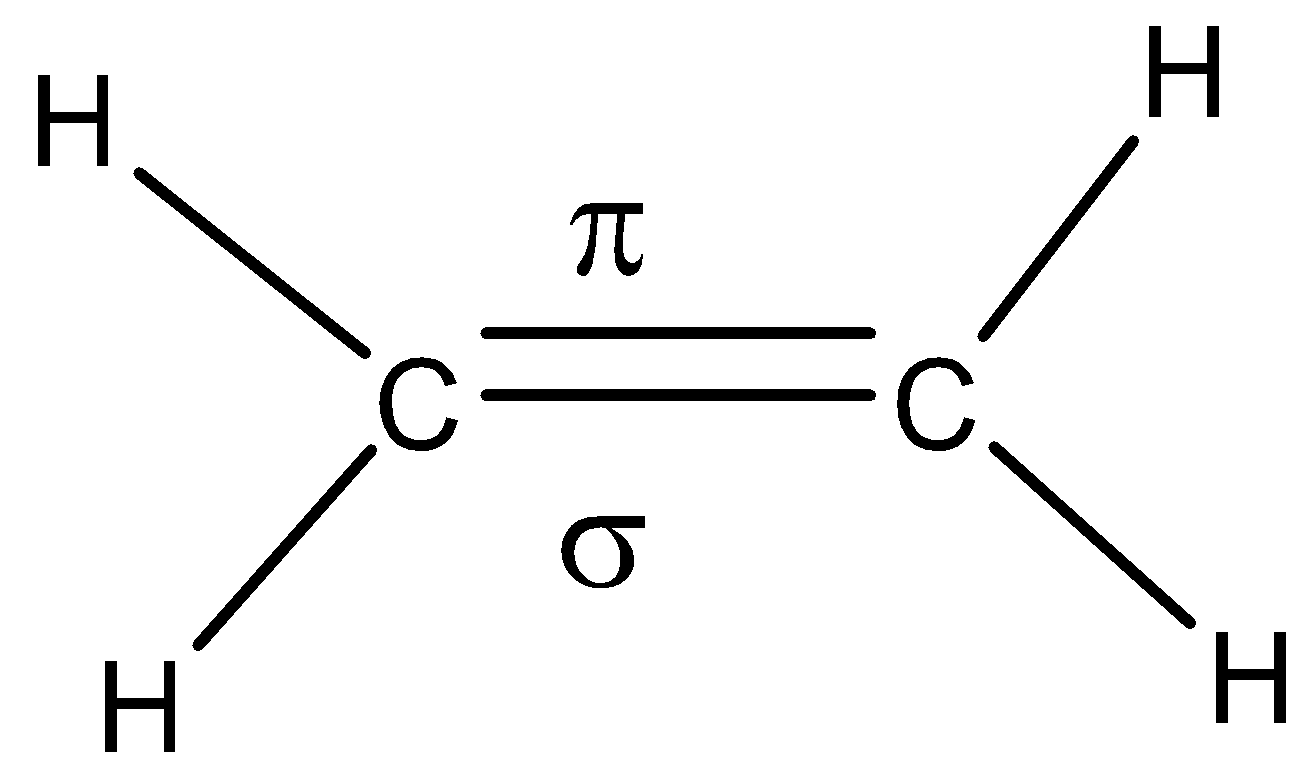
Thus, in this way sigma and pi – bonds can be identified.
Note: Note that in the multiple bonded molecules like ethyne, benzyne etc., there is only one sigma bond present by the first overlap and the rest of the overlaps will be of pi – bonds because in a molecule one sigma bond is possible between two atoms and there can be more than one pi – bond.
Complete step by step answer:
In the lower classes of chemistry, we have studied about the basic concepts of the general chemistry part which deals with the facts that include the concept of types of bonds formed between the atoms in a molecule such as ionic bid, covalent bond, coordinate bond etc.
Let us know what sigma and pi bonds are and how it can be identified.
- Sigma and pi – bonds are the two covalent bonds that are formed between the atoms in a molecule according to their valence.
- Since we know that a covalent bond is formed by sharing a single electron from each atom, the first bond formed will be the sigma bond which is formed by the head on overlap of s – s or s – p or p – p along the internuclear axis.
Similarly, pi bonds are formed by the head on overlapping of parallel p orbitals on adjacent atoms.
In an example of ethane molecule, the sigma and pi bonds formed are depicted as below,

Thus, in this way sigma and pi – bonds can be identified.
Note: Note that in the multiple bonded molecules like ethyne, benzyne etc., there is only one sigma bond present by the first overlap and the rest of the overlaps will be of pi – bonds because in a molecule one sigma bond is possible between two atoms and there can be more than one pi – bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




