
Identify a 'Chemical twin' among the following.
A. Zr — Ta
B. Nb — Tc
C. Hf — Re
D. Nb — Ta
Answer
592.8k+ views
Hint: To know whether the elements are chemical twins or not, we should be knowing their positions on the periodic table. Knowing their periodic table positions will give us an idea about the properties they are possessing.
Complete answer:
A pair of elements having similar properties due to similar atomic radii are called chemical twins. Elements which are present in the d-block of the fifth and sixth period are examples of the chemical twins.
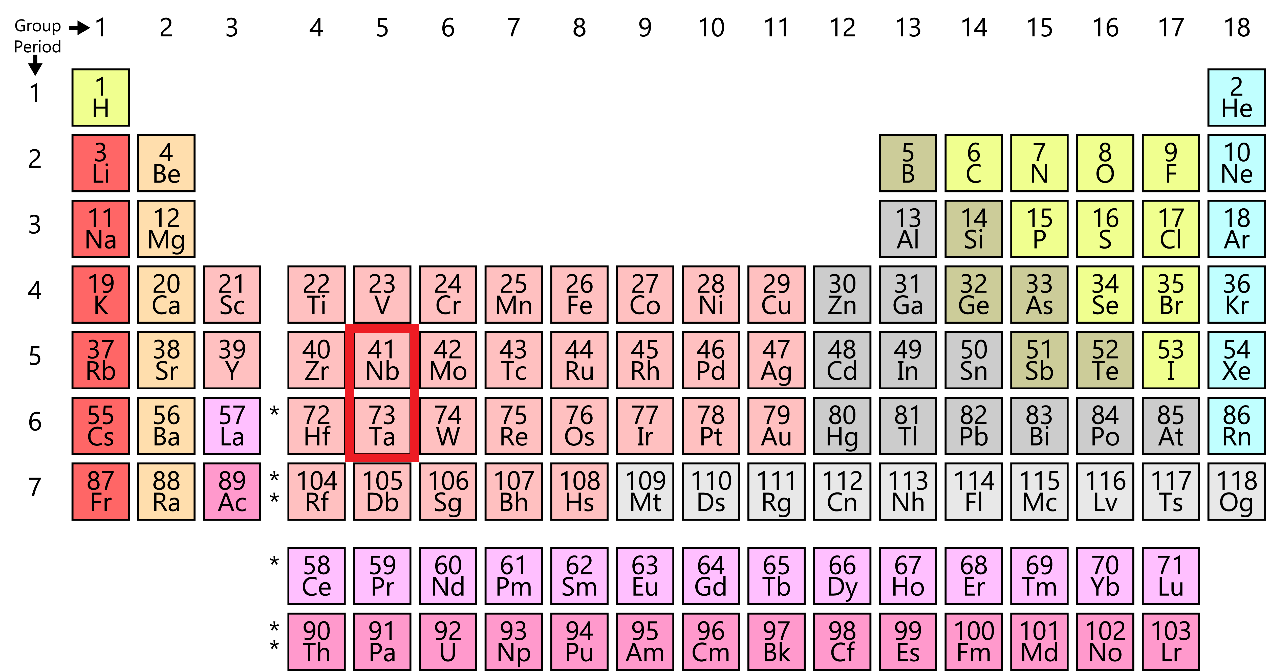
The electronic configuration of niobium is 2, 8, 18, 12, 1. And the electronic configuration of tantalum is 2, 8, 18, 32, 11, 2.
They are said to be chemical twins as the radius increases due to the increase in shells but also decreases due to the increase in effective nuclear charge (force exerted by nucleus on each electron). Thus, both the trends are compensated and the atomic radii are similar. The atomic radius of niobium is 215pm and the atomic radius of tantalum is 220pm.
In this case, Nb — Ta are the elements which are chemical twins
So, the correct answer is “Option D”.
Note: Note that although zirconium and tantalum are diagonally placed in the periodic table, the similarity in diagonally placed elements is only present in period numbers 2 and 3. A vertical relationship of chemical twins is seen in the d-block elements of period 5 and 6. Thus, do not get confused and check the period in which the elements are present and what kind of relationship it indicates before marking your answer.
Complete answer:
A pair of elements having similar properties due to similar atomic radii are called chemical twins. Elements which are present in the d-block of the fifth and sixth period are examples of the chemical twins.

The electronic configuration of niobium is 2, 8, 18, 12, 1. And the electronic configuration of tantalum is 2, 8, 18, 32, 11, 2.
They are said to be chemical twins as the radius increases due to the increase in shells but also decreases due to the increase in effective nuclear charge (force exerted by nucleus on each electron). Thus, both the trends are compensated and the atomic radii are similar. The atomic radius of niobium is 215pm and the atomic radius of tantalum is 220pm.
In this case, Nb — Ta are the elements which are chemical twins
So, the correct answer is “Option D”.
Note: Note that although zirconium and tantalum are diagonally placed in the periodic table, the similarity in diagonally placed elements is only present in period numbers 2 and 3. A vertical relationship of chemical twins is seen in the d-block elements of period 5 and 6. Thus, do not get confused and check the period in which the elements are present and what kind of relationship it indicates before marking your answer.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




