
Identify ‘A’ and ‘B’ in the following reaction?
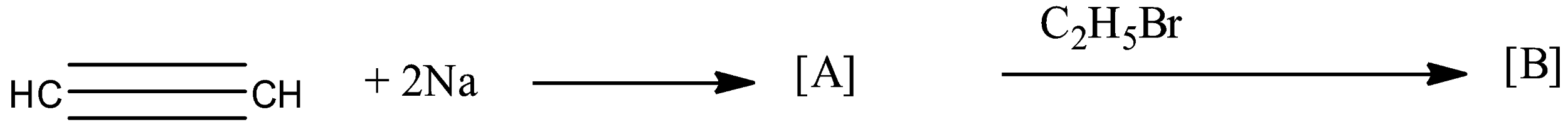
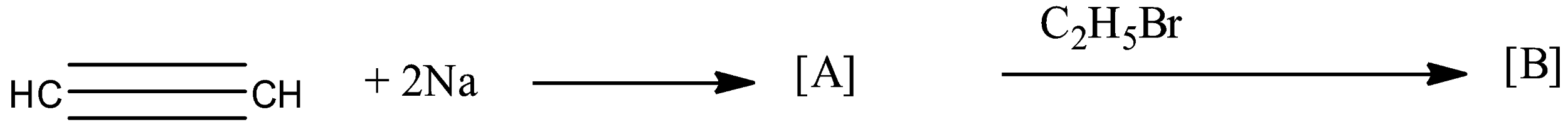
Answer
517.2k+ views
Hint: The reactant in the given reaction of ethyne and the hydrogen atoms in the compound are very reactive due to which they can be easily replaced with the sodium atom. One of the products in the end will be sodium bromide.
Complete answer:
The given reaction in the question is a two-step reaction. The given reaction is:
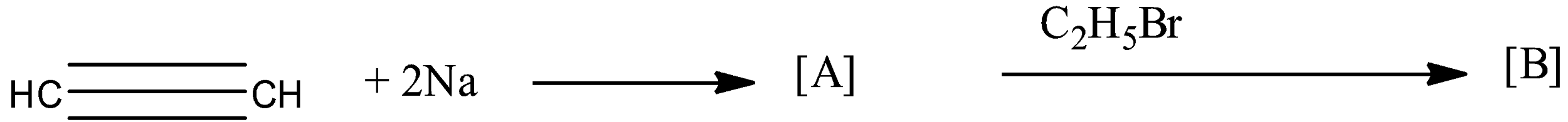
So, the reactant in the reaction is an alkyne because there is a triple bond present between the carbon atoms. Since there are two carbon atoms in the chain then its name will be alkyne. To form [A], the ethyne is treated with sodium elements. As the hydrogen atom, the ethyne is reactive because it is attached with an sp hybridized carbon atom. So, the sodium atom replaces the hydrogen atom in the ethyne, and the formed compound will be monosodium ethynide. Therefore, monosodium ethynide is a compound [A].
Now, this monosodium ethynide is treated with bromoethane. In monosodium ethynide, sodium is the positive part and in bromoethane, bromine is the negative part so, they will combine to form sodium bromide. The main product will be formed when the ethyl molecule will attack the ethynide. This will form But-1-yne. So, the compound [B] will be But-1-yne.
Both the reactions are given below:
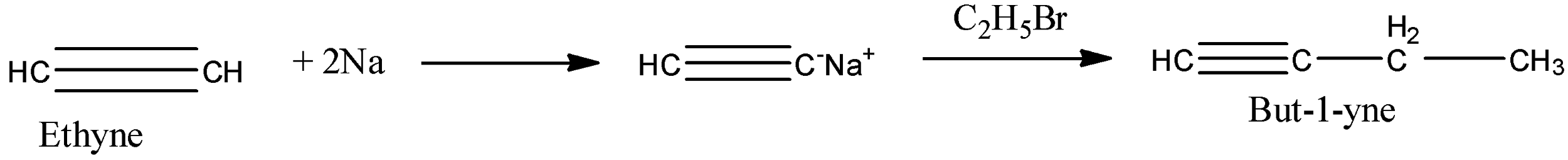
Note:
If you want to replace both the hydrogen atoms in the ethyne then you can treat the compound [A] again with sodium. This is a process in which we can convert the smaller alkynes to larger alkynes easily.
Complete answer:
The given reaction in the question is a two-step reaction. The given reaction is:
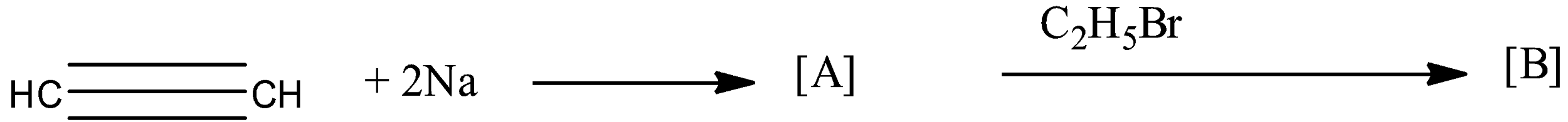
So, the reactant in the reaction is an alkyne because there is a triple bond present between the carbon atoms. Since there are two carbon atoms in the chain then its name will be alkyne. To form [A], the ethyne is treated with sodium elements. As the hydrogen atom, the ethyne is reactive because it is attached with an sp hybridized carbon atom. So, the sodium atom replaces the hydrogen atom in the ethyne, and the formed compound will be monosodium ethynide. Therefore, monosodium ethynide is a compound [A].
Now, this monosodium ethynide is treated with bromoethane. In monosodium ethynide, sodium is the positive part and in bromoethane, bromine is the negative part so, they will combine to form sodium bromide. The main product will be formed when the ethyl molecule will attack the ethynide. This will form But-1-yne. So, the compound [B] will be But-1-yne.
Both the reactions are given below:

Note:
If you want to replace both the hydrogen atoms in the ethyne then you can treat the compound [A] again with sodium. This is a process in which we can convert the smaller alkynes to larger alkynes easily.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




