
When hydrogen peroxide is treated with a cold acidified \[{K_2}C{r_2}{O_7}\] solution containing ether, blue colour is obtained. This is due to:
A. Perchromic acid
B. Potassium chromate
C. Chromium sulphate
D. Chromium trioxide
Answer
581.7k+ views
Hint:Potassium dichromate or \[{K_2}C{r_2}{O_7}\] is an orange coloured oxidizing agent, used in various reactions. On the other hand, hydrogen peroxide or \[{H_2}{O_2}\] is a pale blue viscous liquid. They react with each other and give blue colour compounds.
Complete answer:
As we know, potassium dichromate \[\left( {{K_2}C{r_2}{O_7}} \right)\] is orange in colour and on reaction with pale blue hydrogen peroxide, it yields blue colour compound. To identify the blue colour compound we must write the chemical reaction as follows:
\[{K_2}C{r_2}{O_7} + 4{H_2}{O_2} + {H_2}S{O_4}\xrightarrow{{{\text{Ether}}}}Cr{O_5} + {K_2}S{O_4} + 5{H_2}O\]
The reaction proceeds in three different steps:
Step I: \[{K_2}C{r_2}{O_7} + {H_2}S{O_4} \to {K_2}S{O_4} + {H_2}C{r_2}{O_7}\]
Step II: \[4{H_2}{O_2} \to 4{H_2}O + 4\left( O \right)\]
Step III: \[{H_2}C{r_2}{O_7} + 4\left( O \right) \to 2Cr{O_5} + {H_2}O\]
Hence the three products formed are \[Cr{O_5}\], \[{K_2}S{O_4}\] and \[{H_2}O\]. Among these, \[Cr{O_5}\] or chromium pentoxide has a blue colour in ether.
In the above reaction, the oxidation state of hydrogen peroxide changes from \[ - 1\] to \[ - 2\] as it acts as an oxidizing agent in the reaction.
In less acidic conditions, reaction of hydrogen peroxide with potassium dichromate gives a deep blue-violet coloured compound. Whereas, in an alkaline solution this reaction gives a red-brown coloured compound.
Hence the correct option is A, per-chromic acid.
Additional information:
\[Cr{O_5}\] or chromium pentoxide is also known as perchromic acid and it has a structure of butterfly.
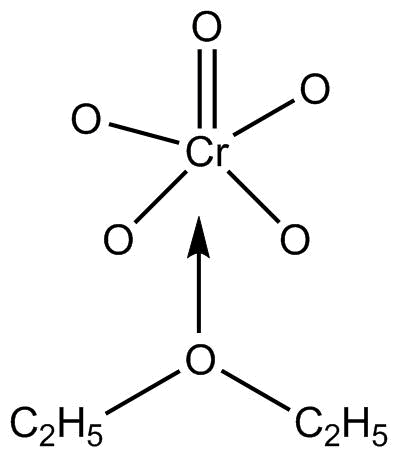
The structure is so, due to two peroxy linkages in the compound.
Note:
Potassium dichromate is an oxidizing agent that oxides aldehydes and alcohols (primary and secondary). It is toxic in nature and also known to be a carcinogen and a mutagen. Therefore, it can be fatal for the environment. It is also a common reason behind dermatitis, especially on arms and hands.
Complete answer:
As we know, potassium dichromate \[\left( {{K_2}C{r_2}{O_7}} \right)\] is orange in colour and on reaction with pale blue hydrogen peroxide, it yields blue colour compound. To identify the blue colour compound we must write the chemical reaction as follows:
\[{K_2}C{r_2}{O_7} + 4{H_2}{O_2} + {H_2}S{O_4}\xrightarrow{{{\text{Ether}}}}Cr{O_5} + {K_2}S{O_4} + 5{H_2}O\]
The reaction proceeds in three different steps:
Step I: \[{K_2}C{r_2}{O_7} + {H_2}S{O_4} \to {K_2}S{O_4} + {H_2}C{r_2}{O_7}\]
Step II: \[4{H_2}{O_2} \to 4{H_2}O + 4\left( O \right)\]
Step III: \[{H_2}C{r_2}{O_7} + 4\left( O \right) \to 2Cr{O_5} + {H_2}O\]
Hence the three products formed are \[Cr{O_5}\], \[{K_2}S{O_4}\] and \[{H_2}O\]. Among these, \[Cr{O_5}\] or chromium pentoxide has a blue colour in ether.
In the above reaction, the oxidation state of hydrogen peroxide changes from \[ - 1\] to \[ - 2\] as it acts as an oxidizing agent in the reaction.
In less acidic conditions, reaction of hydrogen peroxide with potassium dichromate gives a deep blue-violet coloured compound. Whereas, in an alkaline solution this reaction gives a red-brown coloured compound.
Hence the correct option is A, per-chromic acid.
Additional information:
\[Cr{O_5}\] or chromium pentoxide is also known as perchromic acid and it has a structure of butterfly.

The structure is so, due to two peroxy linkages in the compound.
Note:
Potassium dichromate is an oxidizing agent that oxides aldehydes and alcohols (primary and secondary). It is toxic in nature and also known to be a carcinogen and a mutagen. Therefore, it can be fatal for the environment. It is also a common reason behind dermatitis, especially on arms and hands.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

Which out of the following hydrocarbons undergo addition class 11 chemistry CBSE




