
What is the hybridization of a carbon atom in benzene?
Answer
526.5k+ views
Hint :We know that Benzene is the organic compound having the molecular formula hexacarbon hexahydride. It is the ring shaped compound attached to each carbon atom by planar rings. It is also called a hydrocarbon because it has only hydrogen and carbon atoms.
Complete Step By Step Answer:
Benzene is a compound which is also used for the production of polystyrene. In benzene there are formations of triple bonds which are weak due to which it acquires electrophilic character and undergoes reactions with neutrophiles. In benzene each carbon atom is attached to two other carbon atoms, while in non-ring structures the one carbon is attached to one carbon atom. So in total each carbon is attached to two carbon atoms and one hydrogen atom.
So they form hybridization for connecting the atoms by planar rings. The carbon atom forms sigma bonds with other three atoms that are two carbon atoms and one hydrogen atom. So the geometry of each carbon atom in benzene is trigonal planar geometry. In this hybridization it is necessary to achieve the bond angle of $ 120 $ degree and this angle is achieved in benzene. Benzene forms a regular hexagon. It is a stable compound because the lengths of carbon-carbon bonds are somewhere equal either it be the single bond or be the double bond. There is the presence of delocalized electrons above and below the planar ring due to which the benzene becomes stable. In benzene each carbon atom has a p- orbital due to which it can participate in p bonding. Carbon atoms in the benzene ring have a trigonal planar geometry around them since the carry bonds with three other groups and therefore, the hybridization is $ s{{p}^{2}}. $
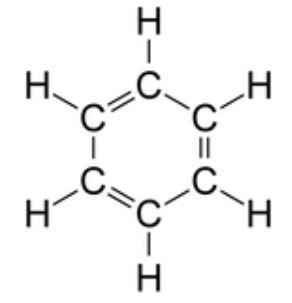
Note :
Remember to know what is hybridization and geometry of each organic compound. They should know the meaning of hybridization. The hybridized orbitals of benzene are not parallel to each other but they overlap laterally to each other for the formation of pi bonds which is not the part of pi electrons.
Complete Step By Step Answer:
Benzene is a compound which is also used for the production of polystyrene. In benzene there are formations of triple bonds which are weak due to which it acquires electrophilic character and undergoes reactions with neutrophiles. In benzene each carbon atom is attached to two other carbon atoms, while in non-ring structures the one carbon is attached to one carbon atom. So in total each carbon is attached to two carbon atoms and one hydrogen atom.
So they form hybridization for connecting the atoms by planar rings. The carbon atom forms sigma bonds with other three atoms that are two carbon atoms and one hydrogen atom. So the geometry of each carbon atom in benzene is trigonal planar geometry. In this hybridization it is necessary to achieve the bond angle of $ 120 $ degree and this angle is achieved in benzene. Benzene forms a regular hexagon. It is a stable compound because the lengths of carbon-carbon bonds are somewhere equal either it be the single bond or be the double bond. There is the presence of delocalized electrons above and below the planar ring due to which the benzene becomes stable. In benzene each carbon atom has a p- orbital due to which it can participate in p bonding. Carbon atoms in the benzene ring have a trigonal planar geometry around them since the carry bonds with three other groups and therefore, the hybridization is $ s{{p}^{2}}. $

Note :
Remember to know what is hybridization and geometry of each organic compound. They should know the meaning of hybridization. The hybridized orbitals of benzene are not parallel to each other but they overlap laterally to each other for the formation of pi bonds which is not the part of pi electrons.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




