
How will you convert aniline to phenol?
Answer
524.5k+ views
Hint: To solve this question we must know the structures of both aniline and phenol. Aniline is an amine group attached to the benzene ring while phenol is the hydroxyl group attached to the benzene ring. The intermediate during this conversion is the benzene diazonium chloride. It is a substitution reaction.
Complete step by step solution:
We know that aniline is amine group $\left( {{\text{N}}{{\text{H}}_{\text{2}}}} \right)$ attached to benzene ring. Aniline is an aromatic compound. Aniline is an industrial chemical. The smell of aniline is like that of a rotten fish. Aniline is highly flammable and burns with smoke. When one hydrogen atom on the benzene ring is replaced by the amine group, aniline is formed. Aniline undergoes substitution reactions.
When aniline is treated with sodium nitride and hydrochloric acid it gives benzene diazonium chloride. The reaction occurs in cold conditions.
The reaction is as follows:

Benzene diazonium chloride on reaction with water forms phenol. This reaction occurs at high temperatures.
Phenol is an aromatic compound. Phenol is used in household products and also for industrial synthesis. In low concentrations, phenol is used as a disinfectant, surgical antiseptic.
The reaction is as follows:
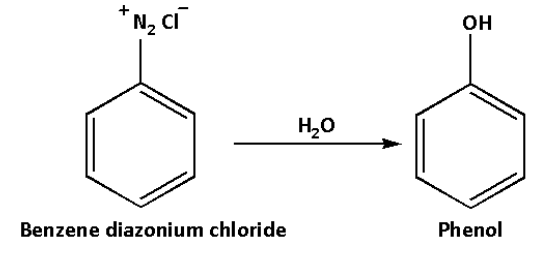
Thus, we can convert aniline to phenol by first treating aniline with sodium nitride and hydrochloric acid which gives benzene diazonium salt which in reaction with water gives phenol.
Note:
In the reaction, phenol is prepared. Thus, it is a preparation reaction of phenol. This reaction is widely used for industrial production of phenol; other methods to produce phenol are from benzene sulphonic acids, from cumene, from haloarenes, etc.
Complete step by step solution:
We know that aniline is amine group $\left( {{\text{N}}{{\text{H}}_{\text{2}}}} \right)$ attached to benzene ring. Aniline is an aromatic compound. Aniline is an industrial chemical. The smell of aniline is like that of a rotten fish. Aniline is highly flammable and burns with smoke. When one hydrogen atom on the benzene ring is replaced by the amine group, aniline is formed. Aniline undergoes substitution reactions.
When aniline is treated with sodium nitride and hydrochloric acid it gives benzene diazonium chloride. The reaction occurs in cold conditions.
The reaction is as follows:

Benzene diazonium chloride on reaction with water forms phenol. This reaction occurs at high temperatures.
Phenol is an aromatic compound. Phenol is used in household products and also for industrial synthesis. In low concentrations, phenol is used as a disinfectant, surgical antiseptic.
The reaction is as follows:

Thus, we can convert aniline to phenol by first treating aniline with sodium nitride and hydrochloric acid which gives benzene diazonium salt which in reaction with water gives phenol.
Note:
In the reaction, phenol is prepared. Thus, it is a preparation reaction of phenol. This reaction is widely used for industrial production of phenol; other methods to produce phenol are from benzene sulphonic acids, from cumene, from haloarenes, etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




