
Here, X is known as Jones reagent, then X is:
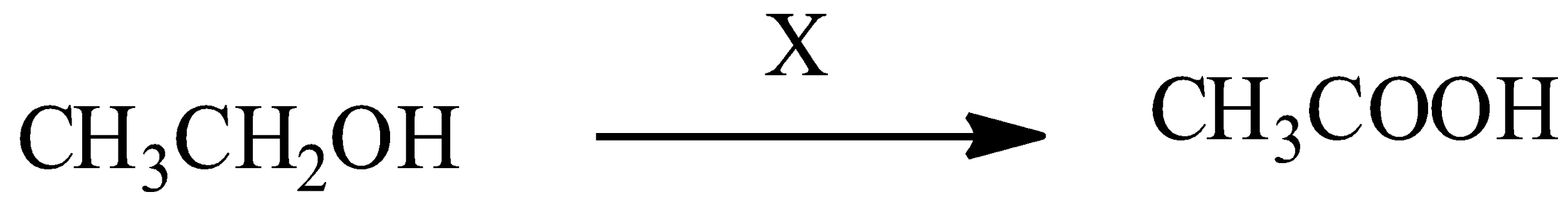
A) $KMn{{O}_{4}}/O{{H}^{-}}$
B) ${{K}_{2}}C{{r}_{2}}{{O}_{7}}/{{H}^{+}}$
C) $Cr{{O}_{3}}-{{H}_{2}}S{{O}_{4}}$
D) $KMn{{O}_{4}}/O{{H}^{+}}$
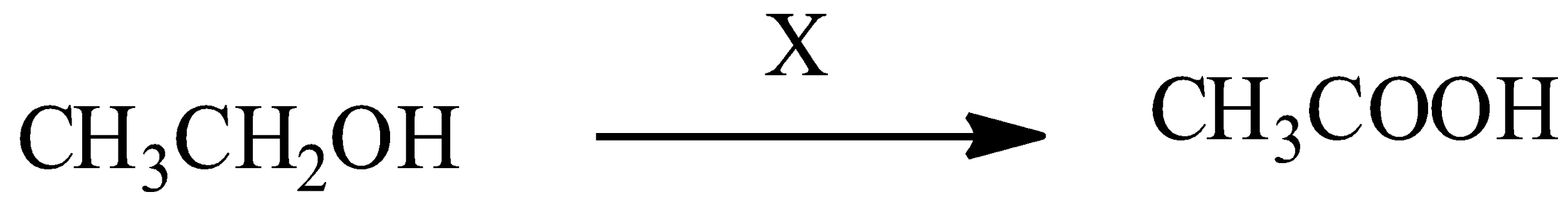
Answer
573k+ views
Hint: The answer is based on the fact that Jones reagent is acidic in nature that acts as an oxidising agent which is strong and oxidises primary and secondary alcohols to carboxylic acids and ketones respectively.
Complete Solution :
In the concepts of organic chemistry, we have studied the chapters that deal with the oxidation, reduction, substitution reaction and also many other reactions.
Now, let us know the basic concept relating to the reactions that include oxidising agents.
- Jones reagent is one of the strong reagents which is used for the oxidation of primary alcohols to the carboxylic acid and secondary alcohols to the ketones.
- Jones reagent is an acidic reagent that is chromium trioxide in sulphuric acid that oxidises primary alcohols to carboxylic acid.
- In the given compound the alcohol given is primary aliphatic acid and is therefore oxidised by Jones reagent to carboxylic acid.
- Jones reagent is given by the composition $Cr{{O}_{3}}+{{H}_{2}}S{{O}_{4}}+{{H}_{2}}O$ and the reaction is called as Jones oxidation reaction.
- Here, acetone is used as a solvent and the oxidation process is exothermic and also rapid.
In this above primary alcohol given, the reaction is as follows:
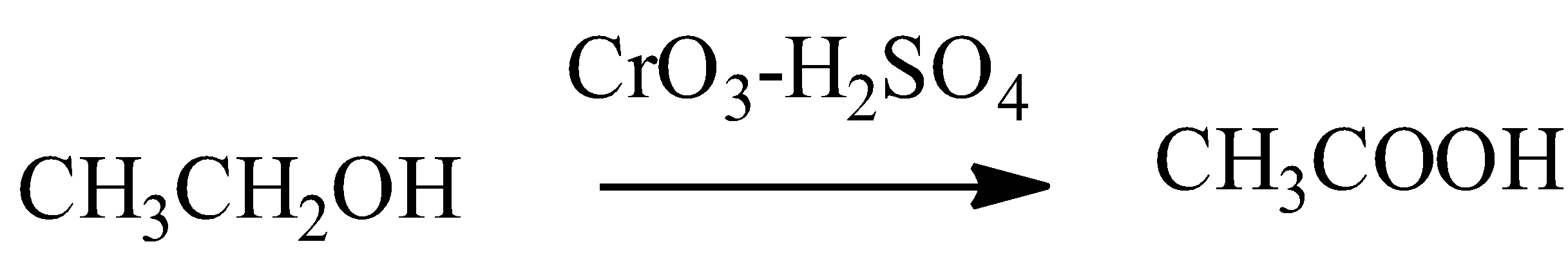
So, the correct answer is “Option C”.
Note: Note that the sulphuric acid present may lead to several side reactions and to reduce this, the amount used can be reduced but the oxidising power of the reagent will decrease in turn. Be thorough with the reagents used for oxidation and reduction of several functional groups.
Complete Solution :
In the concepts of organic chemistry, we have studied the chapters that deal with the oxidation, reduction, substitution reaction and also many other reactions.
Now, let us know the basic concept relating to the reactions that include oxidising agents.
- Jones reagent is one of the strong reagents which is used for the oxidation of primary alcohols to the carboxylic acid and secondary alcohols to the ketones.
- Jones reagent is an acidic reagent that is chromium trioxide in sulphuric acid that oxidises primary alcohols to carboxylic acid.
- In the given compound the alcohol given is primary aliphatic acid and is therefore oxidised by Jones reagent to carboxylic acid.
- Jones reagent is given by the composition $Cr{{O}_{3}}+{{H}_{2}}S{{O}_{4}}+{{H}_{2}}O$ and the reaction is called as Jones oxidation reaction.
- Here, acetone is used as a solvent and the oxidation process is exothermic and also rapid.
In this above primary alcohol given, the reaction is as follows:
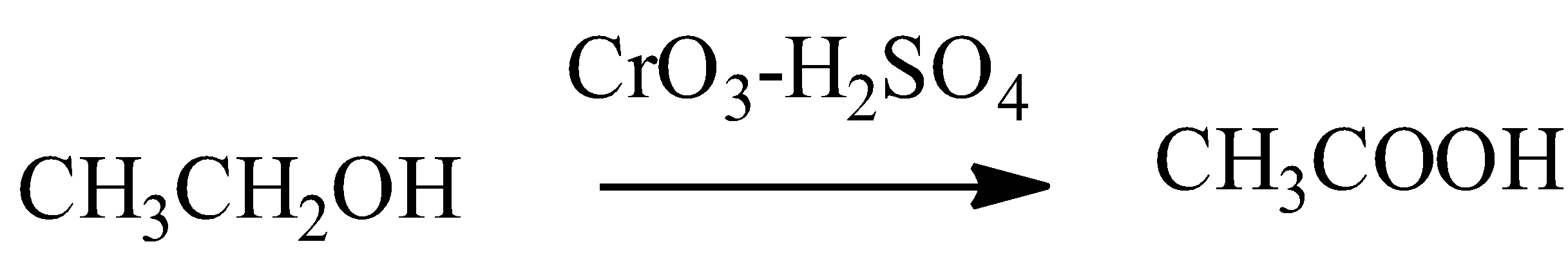
So, the correct answer is “Option C”.
Note: Note that the sulphuric acid present may lead to several side reactions and to reduce this, the amount used can be reduced but the oxidising power of the reagent will decrease in turn. Be thorough with the reagents used for oxidation and reduction of several functional groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




