
\[{\text{HCONHR}}\xrightarrow[{{\text{Pyridine}}}]{{{\text{POC}}{{\text{l}}_{\text{3}}}}}{\text{ (a) + }}{{\text{H}}_{\text{2}}}{\text{O}}\]
In the above reaction is :
A. \[{\text{RCH = NOH}}\]
B. \[{\text{R - N = C = O}}\]
C.
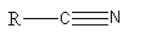
D.

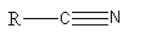

Answer
571.8k+ views
Hint:In the given reaction water is one of the products which indicate it is a dehydration reaction. N-alkyl formamide undergoes a dehydrating reaction with \[{\text{POC}}{{\text{l}}_{\text{3}}}\] in presence of pyridine and gives alkyl isocyanide and water as the products.
Complete answer:
The reaction given to us is:
\[{\text{HCONHR}}\xrightarrow[{{\text{Pyridine}}}]{{{\text{POC}}{{\text{l}}_{\text{3}}}}}{\text{ (a) + }}{{\text{H}}_{\text{2}}}{\text{O}}\]
The structure of the N-alkyl formamide is as follows:
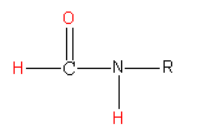
The reagent \[{\text{POC}}{{\text{l}}_{\text{3}}}\] in pyridine act as a dehydrating reagent. It reacts with N-alkyl formamide and eliminates water molecules. In this reaction hydrogen atom bonded to a carbon atom, hydrogen atom bonded to the nitrogen atom and oxygen atom bonded to a carbon atom are eliminated as a water molecule. Due to the loss of water molecules, there is a formation of a triple bond between carbon and nitrogen atoms.
Thus in this reaction, there is a conversion of N-alkyl formamide to alkyl isocyanide.

As there is the loss of water molecule in this reaction and given amide has two hydrogen atoms and one oxygen atom so the product ‘a’ would not contain hydrogen and oxygen atom.
Hence, option (1) \[{\text{RCH = NOH}}\] is incorrect as it contains an oxygen atom. Also, it contains hydrogen atoms bonded to carbon and nitrogen.
Similarly, we can say that option (2) \[{\text{R - N = C = O}}\] is incorrect as it contains oxygen atoms.
Option (3) is incorrect as it has an ‘R’ group bonded to the carbon atom.
Thus, the correct option is (D)
Note:
In this reaction \[{\text{POC}}{{\text{l}}_{\text{3}}}\]act as a dehydrating agent so one of the products of the reaction is water.As given amide contains two hydrogen atoms each bonded to carbon and nitrogen atom and one oxygen atom bonded to carbon. So after the release of the water molecule, another product form will not contain any hydrogen and oxygen atom. So we can easily eliminate the incorrect options that contain hydrogen and oxygen atoms.
Complete answer:
The reaction given to us is:
\[{\text{HCONHR}}\xrightarrow[{{\text{Pyridine}}}]{{{\text{POC}}{{\text{l}}_{\text{3}}}}}{\text{ (a) + }}{{\text{H}}_{\text{2}}}{\text{O}}\]
The structure of the N-alkyl formamide is as follows:

The reagent \[{\text{POC}}{{\text{l}}_{\text{3}}}\] in pyridine act as a dehydrating reagent. It reacts with N-alkyl formamide and eliminates water molecules. In this reaction hydrogen atom bonded to a carbon atom, hydrogen atom bonded to the nitrogen atom and oxygen atom bonded to a carbon atom are eliminated as a water molecule. Due to the loss of water molecules, there is a formation of a triple bond between carbon and nitrogen atoms.
Thus in this reaction, there is a conversion of N-alkyl formamide to alkyl isocyanide.

As there is the loss of water molecule in this reaction and given amide has two hydrogen atoms and one oxygen atom so the product ‘a’ would not contain hydrogen and oxygen atom.
Hence, option (1) \[{\text{RCH = NOH}}\] is incorrect as it contains an oxygen atom. Also, it contains hydrogen atoms bonded to carbon and nitrogen.
Similarly, we can say that option (2) \[{\text{R - N = C = O}}\] is incorrect as it contains oxygen atoms.
Option (3) is incorrect as it has an ‘R’ group bonded to the carbon atom.
Thus, the correct option is (D)
Note:
In this reaction \[{\text{POC}}{{\text{l}}_{\text{3}}}\]act as a dehydrating agent so one of the products of the reaction is water.As given amide contains two hydrogen atoms each bonded to carbon and nitrogen atom and one oxygen atom bonded to carbon. So after the release of the water molecule, another product form will not contain any hydrogen and oxygen atom. So we can easily eliminate the incorrect options that contain hydrogen and oxygen atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




