
What happens when phenol is treated with \[B{r_2}\]?
Answer
518.7k+ views
Hint: When bromine reacts with phenol, a precipitate is formed. Basically, Bromine water \[B{r_2}\], is a mixture of solutions of intense yellow to red colour which has high oxidizing properties. Bromine water is used for the detection of \[(C = C)\] double bonds. We have to find how bromine reacts with phenol and which compound will be formed as precipitate.
Complete answer:
Bromine water has the chemical formula \[(B{r_2})\] and it is also called Bromide bromate solution and bromine solution. It is prepared by dissolving diatomic bromine \[(B{r_2})\] in water \[({H_2}O)\]. It has high oxidizing properties.
The hydroxyl group \[( - OH)\] attached to the benzene in phenol makes the ring more reactive. The oxygen donates its lone pair of electrons to the benzene ring which increases its electron density. The net effect of hydroxyl groups has \[( - OH)\] \[2\],\[4\] - directing groups. The incoming group tends to go at the ortho position and para positions. The phenol undergoes electrophilic substitution reactions.
Bromine water \[(B{r_2})\] test is used for the detection of \[(C = C)\] double bond. In case of alkenes, the colour of the Bromine water decolourises but in case of Phenol, a white precipitate is also formed as well as decolorization takes place.
When phenol is treated with bromine water \[(B{r_2})\], the decolorization of the bromine water takes place i.e. changing of colour from intense yellow-red colour to colorless solution, and a white precipitate is obtained.
The chemical reaction that occurs is as follows:
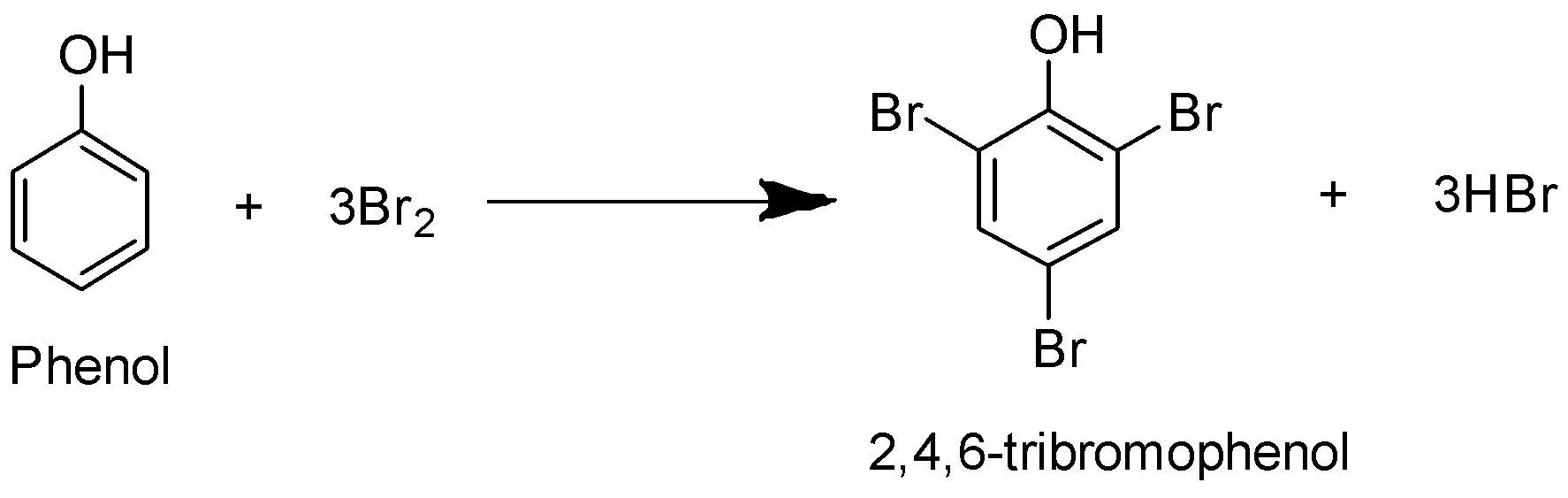
A white precipitate is obtained as of \[2\],\[4\], \[6\]-tribromophenol.
Note:
Bromine water test \[(B{r_2})\] is also called a saturation test. It is a qualitative test used to distinguish between alkanes and alkenes. Remember alkanes do not react with Bromine water and the colour of bromine water remains whereas alkenes undergo an additional reaction in the dark condition and decolorizes the bromine water. The most common compounds that undergo bromine water test are alkenes, glucose, aniline, phenols, enols and acetyl group.
Complete answer:
Bromine water has the chemical formula \[(B{r_2})\] and it is also called Bromide bromate solution and bromine solution. It is prepared by dissolving diatomic bromine \[(B{r_2})\] in water \[({H_2}O)\]. It has high oxidizing properties.
The hydroxyl group \[( - OH)\] attached to the benzene in phenol makes the ring more reactive. The oxygen donates its lone pair of electrons to the benzene ring which increases its electron density. The net effect of hydroxyl groups has \[( - OH)\] \[2\],\[4\] - directing groups. The incoming group tends to go at the ortho position and para positions. The phenol undergoes electrophilic substitution reactions.
Bromine water \[(B{r_2})\] test is used for the detection of \[(C = C)\] double bond. In case of alkenes, the colour of the Bromine water decolourises but in case of Phenol, a white precipitate is also formed as well as decolorization takes place.
When phenol is treated with bromine water \[(B{r_2})\], the decolorization of the bromine water takes place i.e. changing of colour from intense yellow-red colour to colorless solution, and a white precipitate is obtained.
The chemical reaction that occurs is as follows:

A white precipitate is obtained as of \[2\],\[4\], \[6\]-tribromophenol.
Note:
Bromine water test \[(B{r_2})\] is also called a saturation test. It is a qualitative test used to distinguish between alkanes and alkenes. Remember alkanes do not react with Bromine water and the colour of bromine water remains whereas alkenes undergo an additional reaction in the dark condition and decolorizes the bromine water. The most common compounds that undergo bromine water test are alkenes, glucose, aniline, phenols, enols and acetyl group.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Coming together federation is practiced in A India class 12 social science CBSE

How was the Civil Disobedience Movement different from class 12 social science CBSE




