
What happens when acetic acid is treated with \[Zn\] metal?
Answer
569.4k+ views
Hint:
Zinc is a reactive metal which leads to displacement / substitution reaction when reacted with acetic acid. Hydrogen is less reactive than Zinc. Zinc is slightly brittle at room temperature and has a blue-silvery appearance.
Complete step by step answer:
First of all let us understand something about acetic acid. Acetic acid has an IUPAC name ethanoic acid with chemical formula ${C_2}{H_4}{O_2}$ . It’s structure is as below:-
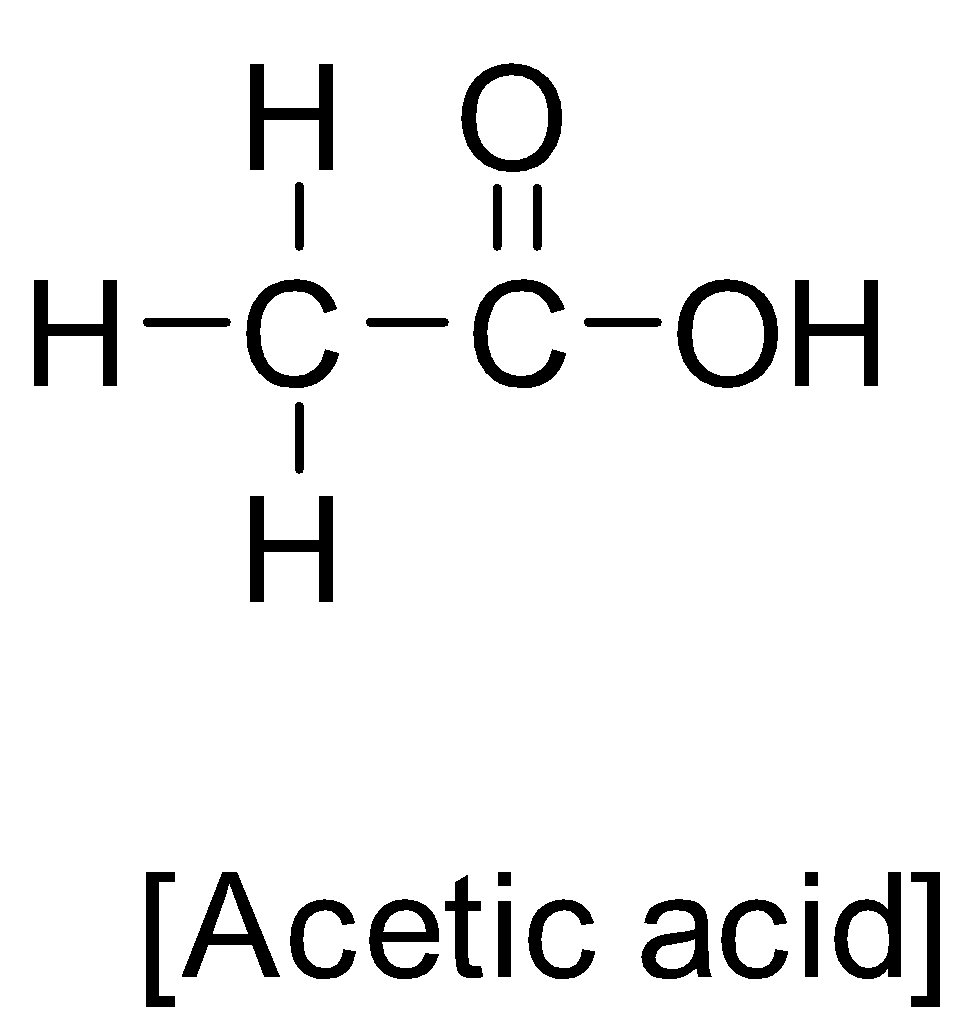
Acetic acid is a colourless liquid organic compound. It is an important industrial chemical and chemical reagent. Acetic acid has a pungent small and has sour taste. It is considered as a weak acid because it did not dissociate completely in water. It is used in making vinegar. Acetic acid is used in the production of esters. Moreover it is also the main component in the formation of acetic anhydride. Acetic acid is also used as an organic solvent/polar solvent. Acetic acid injection is used to treat cancer. Acetic acid is also an antiseptic acid that has a corrosion nature which can cause spinal damage.
Zinc is a reacting element with an atomic number of $30$. Zinc has five stable isotopes and is the ${24^{th}}$ most abundant element in earth’s crust.
When zinc reacts with acetic acid, it leads to the formation of zinc acetate with the evolution of ${H_2}$ gas. The reaction tapes place as
$Zn + 2C{H_3}COOH \to Zn{(C{H_3}COO)_2} + {H_2}$
Note: This reaction does not need any catalyst as the zinc is the transition element and can act as self-catalyst. Moreover in the above reaction, acetic acid having corrosive nature gently corrodes the zinc surface.
Zinc is a reactive metal which leads to displacement / substitution reaction when reacted with acetic acid. Hydrogen is less reactive than Zinc. Zinc is slightly brittle at room temperature and has a blue-silvery appearance.
Complete step by step answer:
First of all let us understand something about acetic acid. Acetic acid has an IUPAC name ethanoic acid with chemical formula ${C_2}{H_4}{O_2}$ . It’s structure is as below:-

Acetic acid is a colourless liquid organic compound. It is an important industrial chemical and chemical reagent. Acetic acid has a pungent small and has sour taste. It is considered as a weak acid because it did not dissociate completely in water. It is used in making vinegar. Acetic acid is used in the production of esters. Moreover it is also the main component in the formation of acetic anhydride. Acetic acid is also used as an organic solvent/polar solvent. Acetic acid injection is used to treat cancer. Acetic acid is also an antiseptic acid that has a corrosion nature which can cause spinal damage.
Zinc is a reacting element with an atomic number of $30$. Zinc has five stable isotopes and is the ${24^{th}}$ most abundant element in earth’s crust.
When zinc reacts with acetic acid, it leads to the formation of zinc acetate with the evolution of ${H_2}$ gas. The reaction tapes place as
$Zn + 2C{H_3}COOH \to Zn{(C{H_3}COO)_2} + {H_2}$
Note: This reaction does not need any catalyst as the zinc is the transition element and can act as self-catalyst. Moreover in the above reaction, acetic acid having corrosive nature gently corrodes the zinc surface.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




