
What is the ground state electronic configuration of the $M{{n}^{2+}}$ ion.
(a) $[Ar]4{{s}^{1}}3{{d}^{5}}$
(b) $[Ar]4{{s}^{2}}3{{d}^{3}}$
(c) $[Ar]3{{d}^{5}}$
(d) $[Ar]3{{d}^{4}}$
Answer
573.6k+ views
Hint: $M{{n}^{2+}}$ ion indicates that the manganese atom loses two electrons from its valence shell and to write the electronic configuration of the manganese ion, we should first know about the electronic configuration of the manganese atom and then by removing the two electrons from the s-orbital , we will get the electronic configuration of the $M{{n}^{2+}}$ ion. Now solve it.
Complete answer:
In the modern periodic table, the physical and chemical properties of the elements are the periodic functions of their atomic weights and the long period table has been divided into the periods (horizontal rows) and groups (vertical columns). In terms of the electron configuration, a group consists of a series of elements which have the same outermost electronic configurations but they have different total numbers of electrons. In the periodic table, there are a total 18 groups and 7 periods in all. The whole elements in the periodic table has been divided into the four blocks i.e. s, p ,d and f- blocks elements. The given manganese ion belongs to the d-block elements and here the last electron enters the d orbital. It occupies the 4 th period and 7th group of the periodic table. It has an atomic number as 25 and mass number as 54. In the outermost valence shell, the manganese atom has six electrons in the d-orbital and two electrons in the s-orbital and the ground state electronic configuration of manganese atom in the ground state is as: $[Ar]4{{s}^{2}}3{{d}^{5}}$.
After the loss of two electrons from the 4s orbital, manganese acquires positive charge i.e. $M{{n}^{2+}}$ and the electronic configuration of the manganese ion in the ground state is as: $[Ar]3{{d}^{5}}$.
Hence, the ground state electronic configuration of the $M{{n}^{2+}}$ ion is $[Ar]3{{d}^{5}}$.
So, option (c) is correct.
Note: When we write the electronic configuration of any element, the electrons are filled according to the Aufbau principle i.e. the electrons are first filled in the orbitals having lower energy followed by orbitals having higher energy as indicated by the arrows.
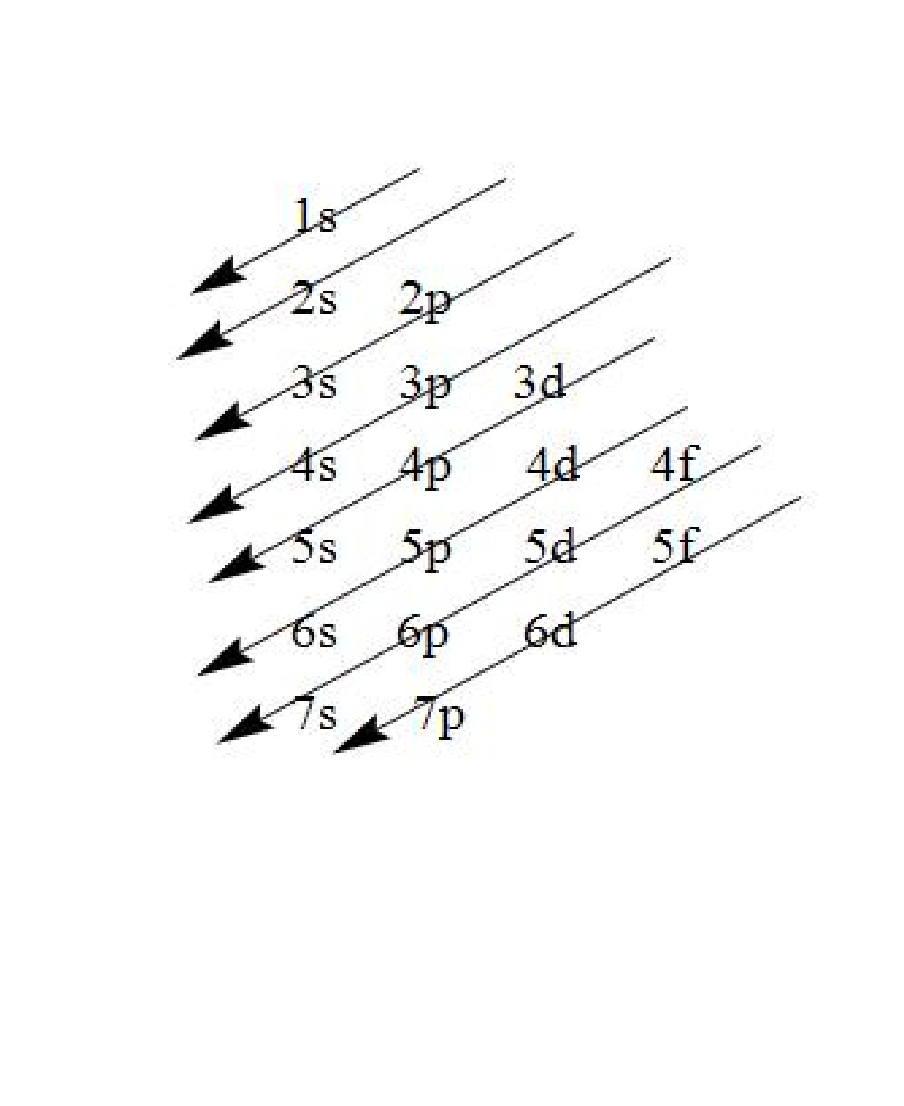
Complete answer:
In the modern periodic table, the physical and chemical properties of the elements are the periodic functions of their atomic weights and the long period table has been divided into the periods (horizontal rows) and groups (vertical columns). In terms of the electron configuration, a group consists of a series of elements which have the same outermost electronic configurations but they have different total numbers of electrons. In the periodic table, there are a total 18 groups and 7 periods in all. The whole elements in the periodic table has been divided into the four blocks i.e. s, p ,d and f- blocks elements. The given manganese ion belongs to the d-block elements and here the last electron enters the d orbital. It occupies the 4 th period and 7th group of the periodic table. It has an atomic number as 25 and mass number as 54. In the outermost valence shell, the manganese atom has six electrons in the d-orbital and two electrons in the s-orbital and the ground state electronic configuration of manganese atom in the ground state is as: $[Ar]4{{s}^{2}}3{{d}^{5}}$.
After the loss of two electrons from the 4s orbital, manganese acquires positive charge i.e. $M{{n}^{2+}}$ and the electronic configuration of the manganese ion in the ground state is as: $[Ar]3{{d}^{5}}$.
Hence, the ground state electronic configuration of the $M{{n}^{2+}}$ ion is $[Ar]3{{d}^{5}}$.
So, option (c) is correct.
Note: When we write the electronic configuration of any element, the electrons are filled according to the Aufbau principle i.e. the electrons are first filled in the orbitals having lower energy followed by orbitals having higher energy as indicated by the arrows.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




