
Gold number is a measure of:
A.The amount of gold present in colloidal solution
B.The amount of gold required to break the colloid
C.The amount of gold requires to protect the colloid
D.None of these
Answer
602.4k+ views
Hint: Gold number is the measure of efficiency to protect a colloidal solution from precipitation. If smaller the gold number value, higher the protective power of the colloid.
Complete answer:
Gold number: It can be defined as the number of milligrams of the protective colloid required to just prevent the precipitation of 10 cc of a gold sol by the addition of 10% NaCl solution.
Gold number is the degree of the minimum amount of lyophilic protective colloid coated (in mg.) on lyophobic particles to prevent the precipitation as shown in figure.
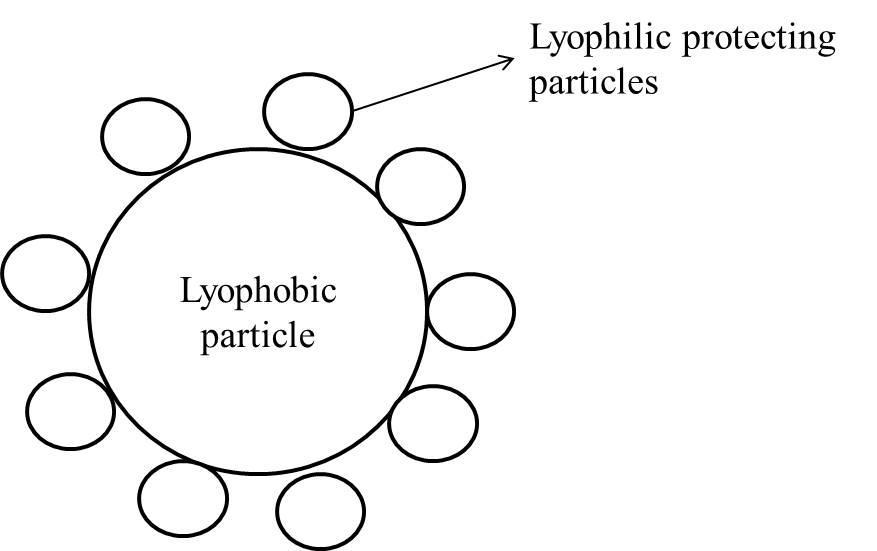
All the given options (A, B, and C) are not matching with the definition of the gold number.
Therefore the correct option is D, None of these.
Additional information:
From the above table we can say that Gelatin has higher protective power of the colloid because the gold number for gelatin is too less compared to other protective colloids.
Note: The influence of the hydrophilic colloid to stop the precipitation of a lyophobic colloid by the addition of an electrolyte depends upon the nature of the colloidal solution. The protective character of many hydrophilic substances can be stated quantitatively by using gold numbers.
Complete answer:
Gold number: It can be defined as the number of milligrams of the protective colloid required to just prevent the precipitation of 10 cc of a gold sol by the addition of 10% NaCl solution.
Gold number is the degree of the minimum amount of lyophilic protective colloid coated (in mg.) on lyophobic particles to prevent the precipitation as shown in figure.

All the given options (A, B, and C) are not matching with the definition of the gold number.
Therefore the correct option is D, None of these.
Additional information:
| Protective colloid | Gold number (in mg/cL) |
| Gelatin | 0.005 - 0.01 |
| Haemoglobin | 0.03 – 0.07 |
| Potato Starch | 20-25 |
| Gum Arabic | 0.15 - 0.25 |
| Sodium oleate | 1 - 5 |
| Dextrin | 125 - 150 |
From the above table we can say that Gelatin has higher protective power of the colloid because the gold number for gelatin is too less compared to other protective colloids.
Note: The influence of the hydrophilic colloid to stop the precipitation of a lyophobic colloid by the addition of an electrolyte depends upon the nature of the colloidal solution. The protective character of many hydrophilic substances can be stated quantitatively by using gold numbers.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

The largest wind power cluster is located in the state class 11 social science CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

Which among the following are examples of coming together class 11 social science CBSE




