
Glycerol is a:
a.) Monofunctional compound
b.) Difunctional compound
c.) Trifunctional compound
d.) Non – functional compound
Answer
603.3k+ views
Hint: Glycerol has three hydroxyl groups. Functionality of a compound is the presence of functional groups. All the hydroxyl groups are attached to three consecutive carbons.
Complete step by step solution:
Functionality is the presence of functional groups in a molecule. Functionality of a molecule has a decisive influence on its reactivity. A monofunctional molecule possesses one function, a difunctional two, a trifunctional three, etc.
Glycerol is also known as glycerine or glycerin. It is a simple polyol compound. It is a colourless, odourless, viscous liquid and is sweet-tasting and non-toxic. The backbone of glycerol is found in lipids known as glycerides. It is also used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Due to the presence of three hydroxyl groups, glycerol is miscible in water and hygroscopic in nature. Glycerol is an achiral compound.
The molecular formula of glycerol is \[{C_3}{H_5}{(OH)_3}\]. The structure of glycerol is:
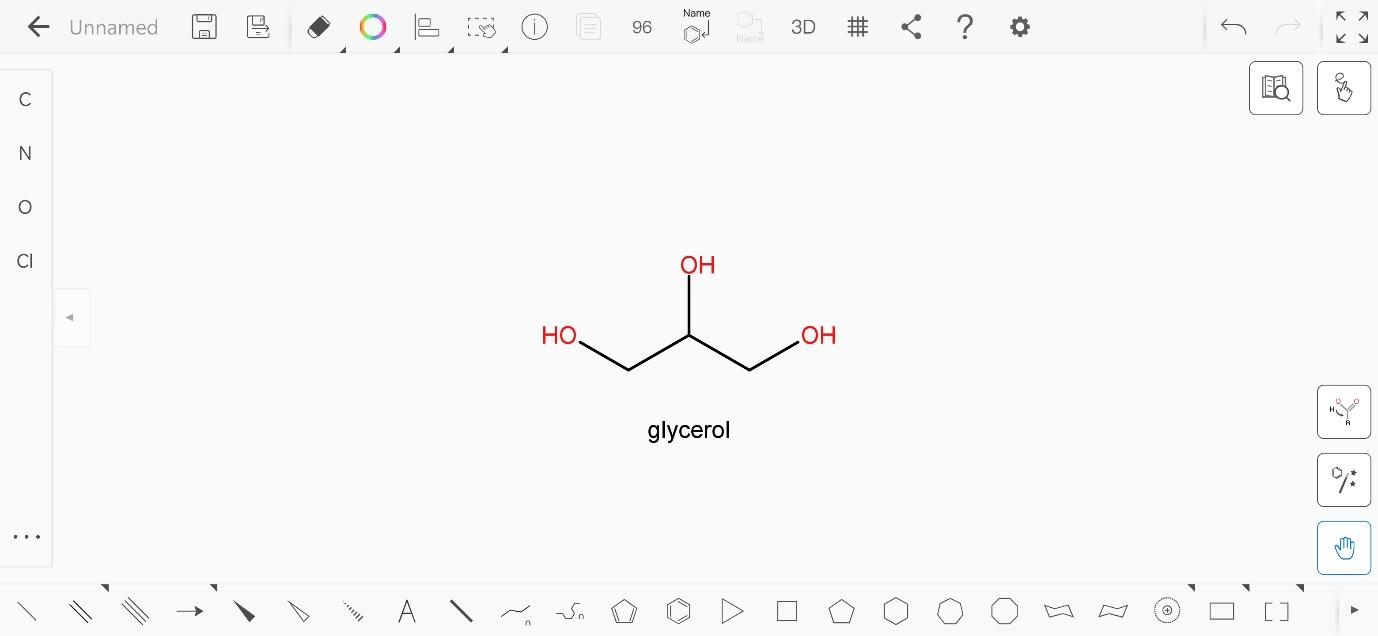
It has three carbons, each of which has one hydroxyl group attached to them. Hence, it has a total of three hydroxyl groups. Thus, glycerol is a trifunctional compound.
Hence, the correct answer is (C).
Note: Remember that the total number of functional groups are counted for the functionality of a compound, not the total distinct functional groups.
Complete step by step solution:
Functionality is the presence of functional groups in a molecule. Functionality of a molecule has a decisive influence on its reactivity. A monofunctional molecule possesses one function, a difunctional two, a trifunctional three, etc.
Glycerol is also known as glycerine or glycerin. It is a simple polyol compound. It is a colourless, odourless, viscous liquid and is sweet-tasting and non-toxic. The backbone of glycerol is found in lipids known as glycerides. It is also used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Due to the presence of three hydroxyl groups, glycerol is miscible in water and hygroscopic in nature. Glycerol is an achiral compound.
The molecular formula of glycerol is \[{C_3}{H_5}{(OH)_3}\]. The structure of glycerol is:

It has three carbons, each of which has one hydroxyl group attached to them. Hence, it has a total of three hydroxyl groups. Thus, glycerol is a trifunctional compound.
Hence, the correct answer is (C).
Note: Remember that the total number of functional groups are counted for the functionality of a compound, not the total distinct functional groups.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




