
Glucose on reaction with $B{{r}_{2}}$ water gives:
Answer
602.4k+ views
Hint: The Fischer projection of glucose molecule reveals that there is an aldehyde group at one of the ends which can be easily oxidized. On the other hand, $B{{r}_{2}}$water is considered as a mild oxidizing agent that can oxidize aldehyde groups to carboxylic acid.
Complete answer:
Glucose is the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose in cell walls, which is the most abundant carbohydrate.
Let us draw the structure of glucose to see the functional groups present in the molecule
Fischer projection of glucose:
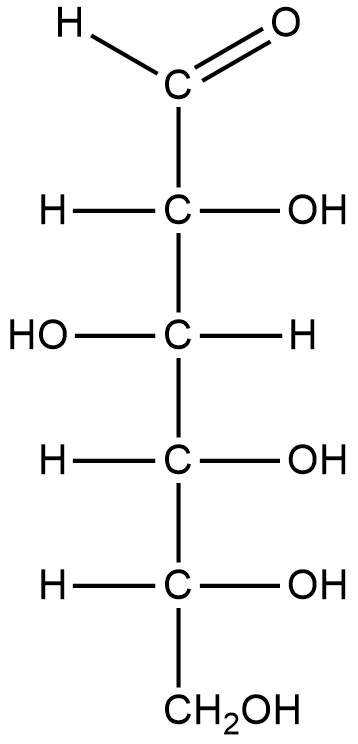
From the above structure we can identify 5 hydroxyl groups and 1 aldehyde group.
$B{{r}_{2}}$ water is widely used to detect unsaturation in a compound. The discoloration of $B{{r}_{2}}$ water indicates unsaturation in the compound.
However, $B{{r}_{2}} $ water is also considered as a mild oxidizing agent. $B{{r}_{2}}$ water oxidizes aldehydes into carboxylic acids. They do not react with ketone due to steric hindrance.
Let us write down the reaction of glucose molecules with $B{{r}_{2}}$ water.
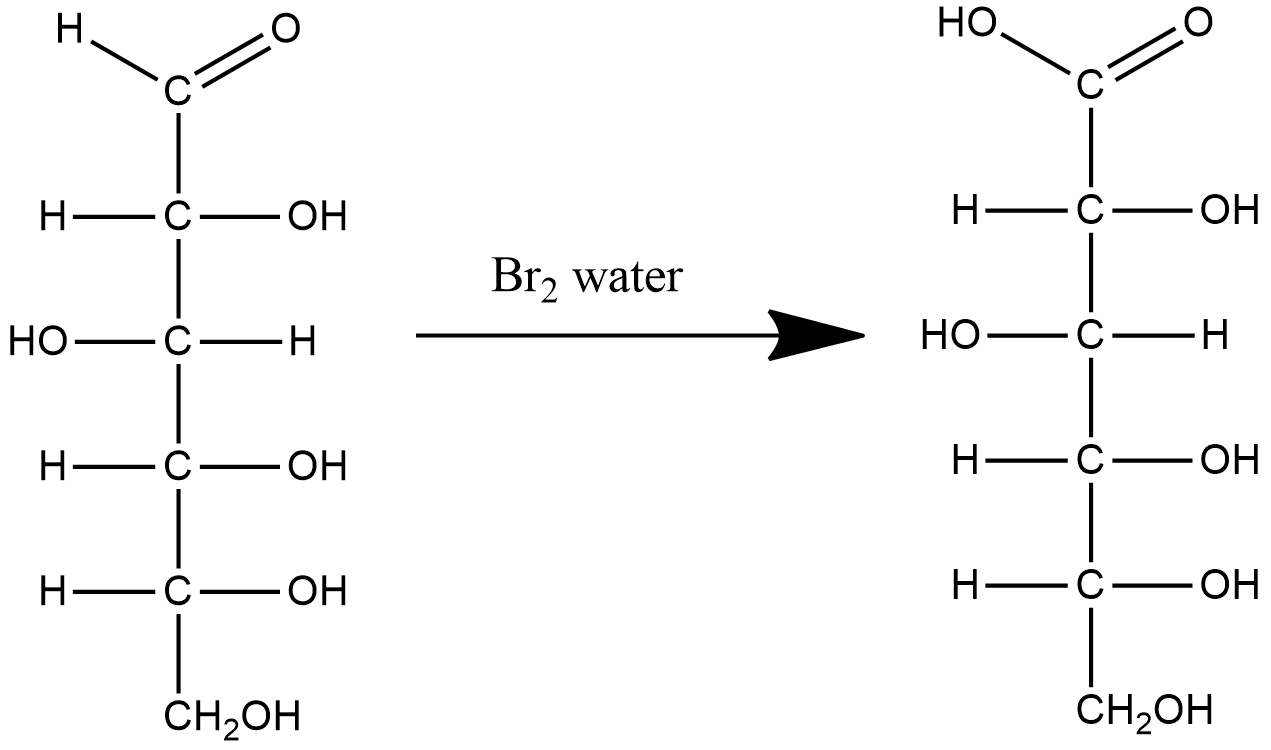
In the above reaction we see that $B{{r}_{2}}$ water oxidizes the aldehyde group present in glucose to carboxylic acid. The product of this reaction is gluconic acid.
Therefore, the correct answer is option (B).
Note: Glucose when subjected to a strong oxidizing agent, gives saccharic acid the product which has a carboxylic acid group on each end of the molecule. The terminal hydroxyl group does not get oxidized as $B{{r}_{2}}$ water is a mild oxidizing agent and cannot oxidize hydroxyl groups.
Complete answer:
Glucose is the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose in cell walls, which is the most abundant carbohydrate.
Let us draw the structure of glucose to see the functional groups present in the molecule
Fischer projection of glucose:

From the above structure we can identify 5 hydroxyl groups and 1 aldehyde group.
$B{{r}_{2}}$ water is widely used to detect unsaturation in a compound. The discoloration of $B{{r}_{2}}$ water indicates unsaturation in the compound.
However, $B{{r}_{2}} $ water is also considered as a mild oxidizing agent. $B{{r}_{2}}$ water oxidizes aldehydes into carboxylic acids. They do not react with ketone due to steric hindrance.
Let us write down the reaction of glucose molecules with $B{{r}_{2}}$ water.

In the above reaction we see that $B{{r}_{2}}$ water oxidizes the aldehyde group present in glucose to carboxylic acid. The product of this reaction is gluconic acid.
Therefore, the correct answer is option (B).
Note: Glucose when subjected to a strong oxidizing agent, gives saccharic acid the product which has a carboxylic acid group on each end of the molecule. The terminal hydroxyl group does not get oxidized as $B{{r}_{2}}$ water is a mild oxidizing agent and cannot oxidize hydroxyl groups.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

The largest wind power cluster is located in the state class 11 social science CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

Which among the following are examples of coming together class 11 social science CBSE




