
How glucose is prepared from cane sugar? Write the formula of the complex Copper (II) hexacyanoferrate (II).
Answer
595.5k+ views
Hint: Cane sugar is sucrose which is a disaccharide composed of one unit of glucose and one unit of fructose that are bound together through an oxygen linkage. Copper (II) hexacyanoferrate (II) is an IUPAC name which follows the guidelines set by the IUPAC. By following these rules, we can write the formula for this compound.
Complete step by step solution:
Sucrose is also called cane sugar. Sucrose is a disaccharide i.e. it is made from two monosaccharide units (glucose and fructose). Its molecular formula is ${ C }_{ 12 }{ H }_{ 22 }{ O }_{ 11 }$, composed of glucose ($\alpha $-D-Glucose) and fructose ($\beta $-D-Fructose) units that are linked together through an oxygen atom. Its structure is given below:
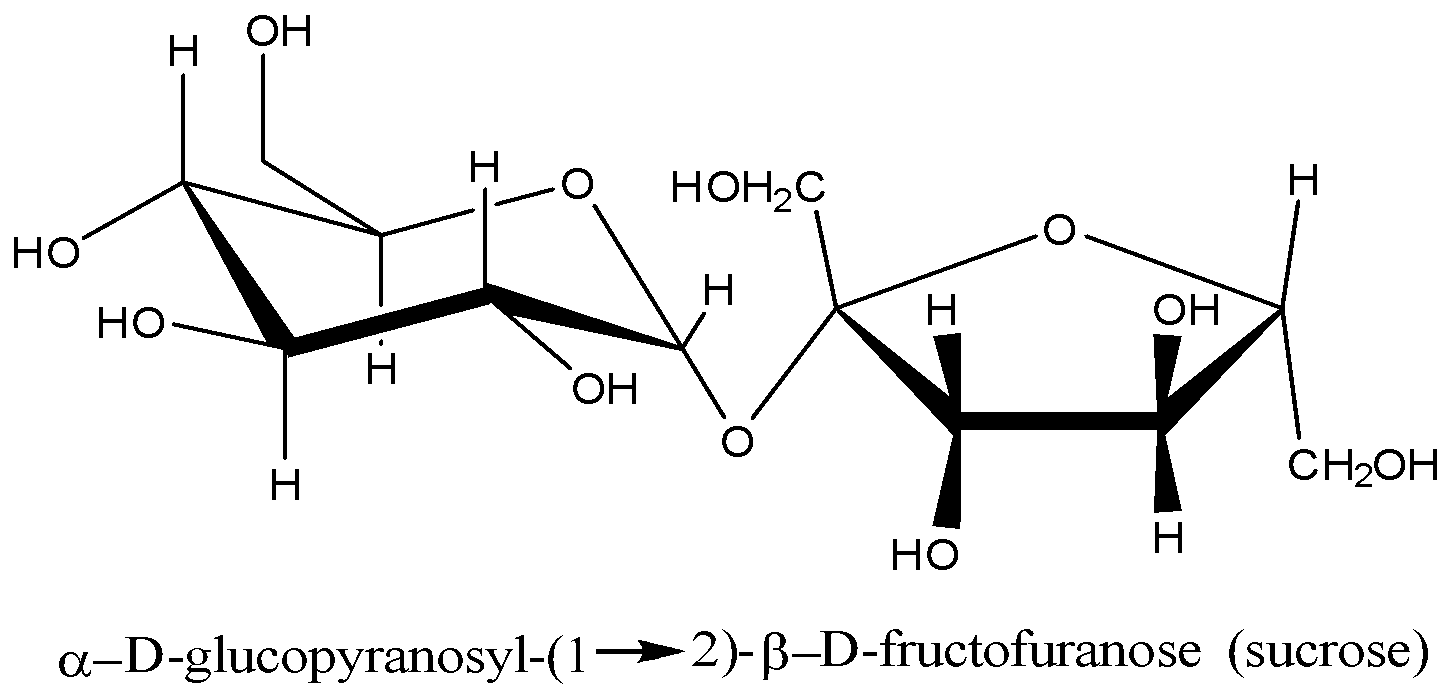
When sucrose is boiled with dilute hydrochloric acid or sulphuric acid, hydrolysis reaction takes place due to which the molecule of sucrose breaks into its monomer units. The hydrolysis reaction of sucrose is shown below:
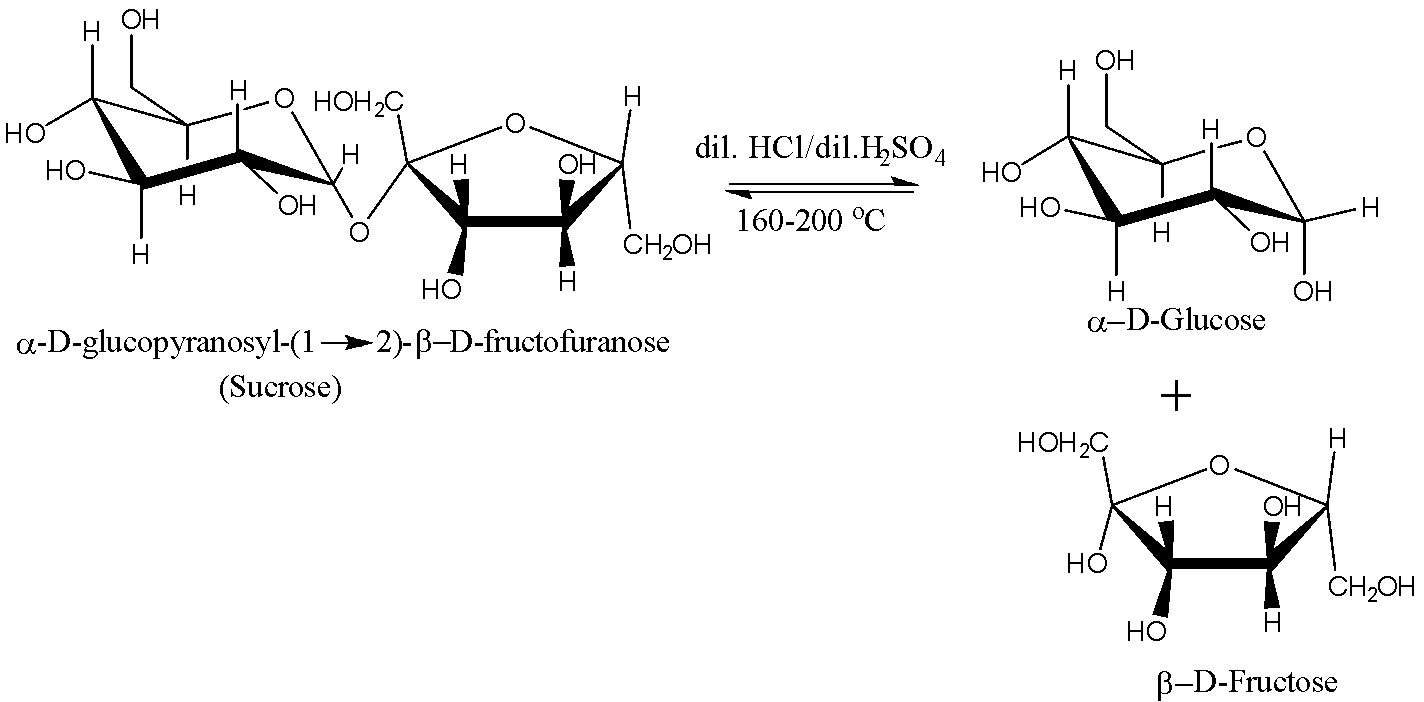
After the completion of the reaction, alcohol is added to the solution and it is kept aside for cooling. Glucose is insoluble in alcohol; therefore it crystallizes first and its crystals are separated by filtration.
Now let us solve the second part of the question:
Copper (II) hexacyanoferrate (II) is an IUPAC name. In order to write the formula of a coordination compound when its IUPAC name is given, certain rules must be followed:
-We need to know the number of cations and the number of anions in the compound for which we will have to compute the charge on each type of ion. The ion is a simple ion (cation or anion) behaves like a free ion and therefore has the usual charge as one radical. Charge on the complex ion (the ion that will be enclosed in square brackets) will be equal to the charge on the central metal atom (which will be equal to its oxidation state) plus the total charge on the coordinated ligands.
-The total charge on a coordination compound as a whole is zero or neutral, therefore total positive charge is equal to the total negative charge. Multiply the cations and the anions by constants such that the charges become equal to each other. The constants by which they are multiplied give us the number of each type of ion present in the compound.
By keeping these rules in mind, let us write the formula for Copper (II) hexacyanoferrate (II).
In this formula, the cation is the simple ion and its oxidation number is +2.
Cation (simple ion) =$ { Cu }^{ 2+ }$
The coordination entity (the anion in this case) is the complex ion. We will write the symbol of the symbol of the metal atom and then the ligands in the alphabetical order:
Anion (complex ion)=$ \left[ Fe(CN{ ) }_{ 6 } \right] ^{ x }$
Where ‘x’ is the total charge on the anion. Since the charge on the anion should be equal to the sum of the charge on the metal atom (iron) and the ligand (cyanide):
x=+2+[$ 6\times (-1)$]=-4
Since the charge on the anion must be equal to the charge on the cation, therefore the charge on the copper cation has to be multiplied by 2 and therefore there are actually two copper cations:
$ 2\times \begin{matrix} { Cu }^{ 2+ } \\ charge=+4 \end{matrix}=\begin{matrix} [Fe{ (CN) }_{ 6 }{ ] }^{ 4- } \\ charge=-4 \end{matrix}\quad $
Hence the formula of Copper (II) hexacyanoferrate (II) will be $ { Cu }_{ 2 }[Fe{ (CN) }_{ 6 }]$. Sugar is prepared by hydrolysing it with a dilute solution of hydrochloric acid or sulphuric acid.
Note: Glucose can also be prepared by boiling starch or cellulose with dilute sulphuric acid. The reaction is given below:
$ \begin{matrix} ({ C }_{ 6 }{ H }_{ 10 }{ O }_{ 5 }{ ) }_{ n } \\ starch/cellulose \end{matrix}+\begin{matrix} n{ H }_{ 2 }O \\ Water \end{matrix}\xrightarrow [ dil.\quad { H }_{ 2 }{ SO }_{ 4 } ]{ 393K,\quad pressure } \begin{matrix} n{ C }_{ 6 }{ H }_{ 12 }{ O }_{ 6 } \\ Glucose \end{matrix}$
Complete step by step solution:
Sucrose is also called cane sugar. Sucrose is a disaccharide i.e. it is made from two monosaccharide units (glucose and fructose). Its molecular formula is ${ C }_{ 12 }{ H }_{ 22 }{ O }_{ 11 }$, composed of glucose ($\alpha $-D-Glucose) and fructose ($\beta $-D-Fructose) units that are linked together through an oxygen atom. Its structure is given below:

When sucrose is boiled with dilute hydrochloric acid or sulphuric acid, hydrolysis reaction takes place due to which the molecule of sucrose breaks into its monomer units. The hydrolysis reaction of sucrose is shown below:

After the completion of the reaction, alcohol is added to the solution and it is kept aside for cooling. Glucose is insoluble in alcohol; therefore it crystallizes first and its crystals are separated by filtration.
Now let us solve the second part of the question:
Copper (II) hexacyanoferrate (II) is an IUPAC name. In order to write the formula of a coordination compound when its IUPAC name is given, certain rules must be followed:
-We need to know the number of cations and the number of anions in the compound for which we will have to compute the charge on each type of ion. The ion is a simple ion (cation or anion) behaves like a free ion and therefore has the usual charge as one radical. Charge on the complex ion (the ion that will be enclosed in square brackets) will be equal to the charge on the central metal atom (which will be equal to its oxidation state) plus the total charge on the coordinated ligands.
-The total charge on a coordination compound as a whole is zero or neutral, therefore total positive charge is equal to the total negative charge. Multiply the cations and the anions by constants such that the charges become equal to each other. The constants by which they are multiplied give us the number of each type of ion present in the compound.
By keeping these rules in mind, let us write the formula for Copper (II) hexacyanoferrate (II).
In this formula, the cation is the simple ion and its oxidation number is +2.
Cation (simple ion) =$ { Cu }^{ 2+ }$
The coordination entity (the anion in this case) is the complex ion. We will write the symbol of the symbol of the metal atom and then the ligands in the alphabetical order:
Anion (complex ion)=$ \left[ Fe(CN{ ) }_{ 6 } \right] ^{ x }$
Where ‘x’ is the total charge on the anion. Since the charge on the anion should be equal to the sum of the charge on the metal atom (iron) and the ligand (cyanide):
x=+2+[$ 6\times (-1)$]=-4
Since the charge on the anion must be equal to the charge on the cation, therefore the charge on the copper cation has to be multiplied by 2 and therefore there are actually two copper cations:
$ 2\times \begin{matrix} { Cu }^{ 2+ } \\ charge=+4 \end{matrix}=\begin{matrix} [Fe{ (CN) }_{ 6 }{ ] }^{ 4- } \\ charge=-4 \end{matrix}\quad $
Hence the formula of Copper (II) hexacyanoferrate (II) will be $ { Cu }_{ 2 }[Fe{ (CN) }_{ 6 }]$. Sugar is prepared by hydrolysing it with a dilute solution of hydrochloric acid or sulphuric acid.
Note: Glucose can also be prepared by boiling starch or cellulose with dilute sulphuric acid. The reaction is given below:
$ \begin{matrix} ({ C }_{ 6 }{ H }_{ 10 }{ O }_{ 5 }{ ) }_{ n } \\ starch/cellulose \end{matrix}+\begin{matrix} n{ H }_{ 2 }O \\ Water \end{matrix}\xrightarrow [ dil.\quad { H }_{ 2 }{ SO }_{ 4 } ]{ 393K,\quad pressure } \begin{matrix} n{ C }_{ 6 }{ H }_{ 12 }{ O }_{ 6 } \\ Glucose \end{matrix}$
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




