
Give the IUPAC name of the following compounds:
Ethylene.
Answer
565.2k+ views
Hint: IUPAC nomenclature can be defined as the system used for naming the organic compounds in the chemistry. It stands for “International Union of Pure and Applied Chemistry.” IUPAC is known for the systematic approach as there are some rules which must be followed to name a compound like longest chain rule, naming the substituents attached to the chain. This could be easier with the help of structure.
Complete step by step answer:
Now, first we will see the structure of compound ethylene represented as follows:
$C{H_2} = C{H_2}$ or
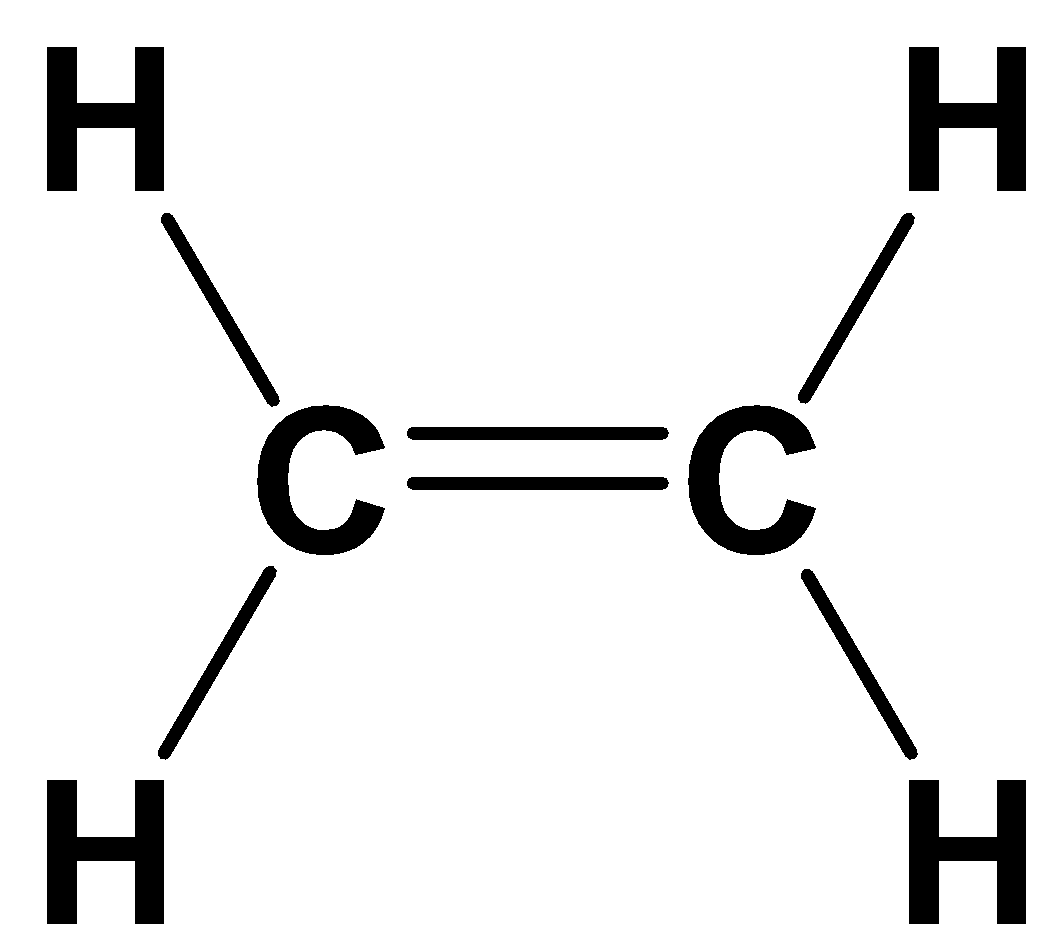
From the structure, the chemical formula of ethylene identified is ${C_2}{H_4}$. The presence of double bonds in the structure indicates that it belongs to the category of alkenes.
Now, let us see the various rules followed for the naming of the compound
1.Longest chain rule: It states the identification of the longest parent chain and it will lead to the identity of ‘root’ word. Like in this compound there are two carbons attached to hydrogen atoms. So, the presence of two carbon atoms means ‘Eth’.
2.Writing the suffix: While naming the suffix is described in two ways: Primary and secondary. Primary is used in case of alkanes, alkenes, alkynes etc.
Secondary suffix is used in case of compounds having substituents attached to it. For example: when an alcoholic group is attached to an alkane chain then the suffix added is ‘ol’.
Thus, in this case the suffix used will be ‘ene’ as mentioned it belongs to an alkene.
In this case, we can say that the IUPAC name of ethylene is ‘ethene’. Because according to the structure it has no longest chain, equal numbers of hydrogen atoms are bonded to the carbon atoms.
Thus, Ethene is the required IUPAC name.
Note: There are other rules which are followed for more complex compounds like after identification of suffix; the prefix would also be known which is being added before the root name like in case of cyclic compounds prefix used is cyclo. The formula for alkenes can be verified with the help of ${C_n}{H_{2n}}$, or we can say it is another way of naming the compounds like alkenes.
Complete step by step answer:
Now, first we will see the structure of compound ethylene represented as follows:
$C{H_2} = C{H_2}$ or

From the structure, the chemical formula of ethylene identified is ${C_2}{H_4}$. The presence of double bonds in the structure indicates that it belongs to the category of alkenes.
Now, let us see the various rules followed for the naming of the compound
1.Longest chain rule: It states the identification of the longest parent chain and it will lead to the identity of ‘root’ word. Like in this compound there are two carbons attached to hydrogen atoms. So, the presence of two carbon atoms means ‘Eth’.
2.Writing the suffix: While naming the suffix is described in two ways: Primary and secondary. Primary is used in case of alkanes, alkenes, alkynes etc.
Secondary suffix is used in case of compounds having substituents attached to it. For example: when an alcoholic group is attached to an alkane chain then the suffix added is ‘ol’.
Thus, in this case the suffix used will be ‘ene’ as mentioned it belongs to an alkene.
In this case, we can say that the IUPAC name of ethylene is ‘ethene’. Because according to the structure it has no longest chain, equal numbers of hydrogen atoms are bonded to the carbon atoms.
Thus, Ethene is the required IUPAC name.
Note: There are other rules which are followed for more complex compounds like after identification of suffix; the prefix would also be known which is being added before the root name like in case of cyclic compounds prefix used is cyclo. The formula for alkenes can be verified with the help of ${C_n}{H_{2n}}$, or we can say it is another way of naming the compounds like alkenes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




