
Give the IUPAC name of the following compound:
Oxalic acid
Answer
593.1k+ views
Hint:
The international Union of pure and applied chemistry (IUPAC) is an international federation that represents chemists in individual countries. It is the world authority on chemical nomenclature and terminology, including the naming of new elements in the periodic table on standardized methods for measurement.
Complete step by step answer:
-Oxalic acid is an organic compound with the formula \[{C_2}{H_2}{O_4}\]. It is a white crystalline solid that forms a colorless solution in water. Its condensed formula is $HOOCCOOH$, reflecting its classification as the simplest dicarboxylic acid. Its acid strength is much greater than that of acetic acid.
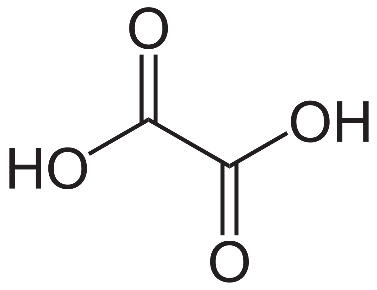
-The IUPAC name of oxalic acid is Ethanedioic acid.
-It is also called ethanedioic acid, a colorless, crystalline, toxic organic compound belonging to the family of carboxylic acids.
-Oxalic acid is classified as a weak acid. It is weaker than ${H_3}{O^ + }$ ion (water). But it is stronger than acetic acid, sulfurous acid, nitrous acid, benzoic acid etc. Firstly, it is an organic compound and generally organic compounds are not strong acids. It is also known as dicarboxylic acid.
Note:
Oxalic acid is an essential household chemical that can be used, like many acids, as a cleaner for various things. For example, as a rust remover, a cleaning agent, on wood work as a stain lifter, as a bleaching agent, and many more. It is toxic because of its acidic and chelating properties. It may cause burns, severe gastroenteritis and vomiting. It is especially toxic when ingested.
The international Union of pure and applied chemistry (IUPAC) is an international federation that represents chemists in individual countries. It is the world authority on chemical nomenclature and terminology, including the naming of new elements in the periodic table on standardized methods for measurement.
Complete step by step answer:
-Oxalic acid is an organic compound with the formula \[{C_2}{H_2}{O_4}\]. It is a white crystalline solid that forms a colorless solution in water. Its condensed formula is $HOOCCOOH$, reflecting its classification as the simplest dicarboxylic acid. Its acid strength is much greater than that of acetic acid.

-The IUPAC name of oxalic acid is Ethanedioic acid.
-It is also called ethanedioic acid, a colorless, crystalline, toxic organic compound belonging to the family of carboxylic acids.
-Oxalic acid is classified as a weak acid. It is weaker than ${H_3}{O^ + }$ ion (water). But it is stronger than acetic acid, sulfurous acid, nitrous acid, benzoic acid etc. Firstly, it is an organic compound and generally organic compounds are not strong acids. It is also known as dicarboxylic acid.
Note:
Oxalic acid is an essential household chemical that can be used, like many acids, as a cleaner for various things. For example, as a rust remover, a cleaning agent, on wood work as a stain lifter, as a bleaching agent, and many more. It is toxic because of its acidic and chelating properties. It may cause burns, severe gastroenteritis and vomiting. It is especially toxic when ingested.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




