
Give the geometrical shapes of the following molecules based on VSEPR theory:
i) \[BeC{l_2}\]
ii) \[B{F_3}\]
iii) \[S{F_6}\]
Answer
552.3k+ views
Hint: VSEPR hypothesis recommends that the mathematical game plan of terminal molecules, or gatherings of particles about a focal iota in a covalent compound, or charged particle, is resolved exclusively by the shocks between electron sets present in the valence shell of the focal molecule.
Complete step by step answer:
Atomic Geometries. The VSEPR hypothesis portrays five fundamental states of straightforward particles: direct, three-sided planar, tetrahedral, three-sided bipyramidal, and octahedral. The VSEPR hypothesis hence sees repugnance by the solitary pair to be more prominent than the aversion by a holding pair. For instance, the $H_2O$ particle has four electron sets in its valence shell: two solitary sets and two bond sets. The four electron sets are spread in order to point generally towards the apices of a tetrahedron.
Geometrical Shape of \[BeC{l_2}\]: 2 bond pair 0 solitary pair SP Hybridization Linear Geometry. Its electronic design is 1s2, 2s2, where two electrons are available in the valence shell. During the development of \[BeC{l_2}\], beryllium particle bonds with two chlorine molecules through single covalent bonds. The quantity of electron sets around the focal molecule will be two. No solitary pair is found in the particle. In the event that we examine this data, at that point we can presume that \[BeC{l_2}\] has sp hybridization.
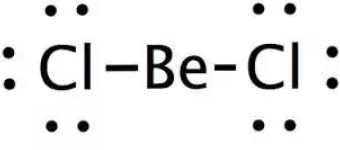
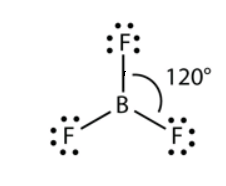
Geometrical Shape of \[B{F_3}\]: 3 bond pair 0 solitary pair \[S{P^2}\] Hybridization Trigonal Geometry. Boron trifluoride holding. The calculation of the \[B{F_3}\] particle is called a three-sided planar. The fluorine molecules are situated at the vertices of a symmetrical triangle. The F-B-F point is 120° and each of the four ions lie in a similar plane.
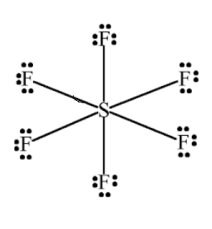
Geometrical Shape of \[s{f_6}\]: 6 bond pair 0 solitary pair \[s{p^3}{d^2}\] Hybridization Square bi pyramidal Geometry: Sulphur hexafluoride has 6 districts of electron thickness around the focal
sulphur particle (6 bonds, no solitary sets). The subsequent shape is an octahedron with 90° F-S-F bond points.
Note: In science, a solitary pair alludes to a couple of valence electrons that are not common with another particle in a covalent bond and is now and then called an unshared pair or non-holding pair. Solitary sets are found in the peripheral electron shell of molecules. They can be recognized by utilizing a Lewis structure.
Complete step by step answer:
Atomic Geometries. The VSEPR hypothesis portrays five fundamental states of straightforward particles: direct, three-sided planar, tetrahedral, three-sided bipyramidal, and octahedral. The VSEPR hypothesis hence sees repugnance by the solitary pair to be more prominent than the aversion by a holding pair. For instance, the $H_2O$ particle has four electron sets in its valence shell: two solitary sets and two bond sets. The four electron sets are spread in order to point generally towards the apices of a tetrahedron.
Geometrical Shape of \[BeC{l_2}\]: 2 bond pair 0 solitary pair SP Hybridization Linear Geometry. Its electronic design is 1s2, 2s2, where two electrons are available in the valence shell. During the development of \[BeC{l_2}\], beryllium particle bonds with two chlorine molecules through single covalent bonds. The quantity of electron sets around the focal molecule will be two. No solitary pair is found in the particle. In the event that we examine this data, at that point we can presume that \[BeC{l_2}\] has sp hybridization.
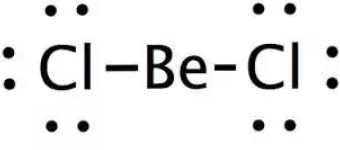
Geometrical Shape of \[B{F_3}\]: 3 bond pair 0 solitary pair \[S{P^2}\] Hybridization Trigonal Geometry. Boron trifluoride holding. The calculation of the \[B{F_3}\] particle is called a three-sided planar. The fluorine molecules are situated at the vertices of a symmetrical triangle. The F-B-F point is 120° and each of the four ions lie in a similar plane.


Geometrical Shape of \[s{f_6}\]: 6 bond pair 0 solitary pair \[s{p^3}{d^2}\] Hybridization Square bi pyramidal Geometry: Sulphur hexafluoride has 6 districts of electron thickness around the focal
sulphur particle (6 bonds, no solitary sets). The subsequent shape is an octahedron with 90° F-S-F bond points.
Note: In science, a solitary pair alludes to a couple of valence electrons that are not common with another particle in a covalent bond and is now and then called an unshared pair or non-holding pair. Solitary sets are found in the peripheral electron shell of molecules. They can be recognized by utilizing a Lewis structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




