
Give the following reactions:
1) Benzoin Condensation
2) Knoevenagel Reaction
Answer
597.6k+ views
Hint:
1) In Benzoin condensation is an additional reaction involving two aldehydes. The reaction generally occurs between two aromatic aldehydes on glyorals. This reaction is catalysed by nucleophilic such as aynaide or ab N – heterocyclic carbon.
2) In Knoevenagel Reaction there is condensation of carbon acid compounds with aldehydes to afford \[\alpha ,\beta \] unsaturated compounds. It is a nucleophilic addition of an active hydrogen compound to a carbonyl group followed by a dehydration reaction in which a water molecule is eliminated and this is why it is known as condensation reaction. Product obtained is a conjugated enone.
Complete answer: 1) Benzoin condensation
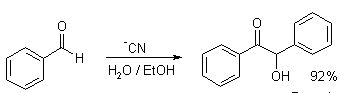
An example of this reaction is when benzaldehyde is refluxed with aqueous alcoholic potassium cyanide then we obtain an \[\alpha -\] hydroxy ketone called benzoin as the product.
2) Knoevenagel Reaction
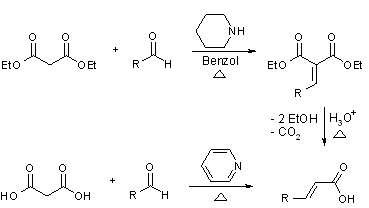
An example of this reaction is when benzaldehyde condenses with malonic acid in presence of pyridine as a basic catalyst. Here malonic acid and the base present generate a carbanion which acts as a nucleophile and attacks on the benzaldehyde.
Note: A condensation reaction is a closure of an organic addition reaction that typically proceeds in a step –wise fashion to produce an addition product, usually in equilibrium and a water molecule is released. In organic chemistry, nucleophilic substitution is a fundamental class of reaction in which a leaving group is replaced by an electron rich compound and the main involved is called substrate.
2) In Knoevenagel Reaction there is condensation of carbon acid compounds with aldehydes to afford \[\alpha ,\beta \] unsaturated compounds. It is a nucleophilic addition of an active hydrogen compound to a carbonyl group followed by a dehydration reaction in which a water molecule is eliminated and this is why it is known as condensation reaction. Product obtained is a conjugated enone.
Complete answer: 1) Benzoin condensation
An example of this reaction is when benzaldehyde is refluxed with aqueous alcoholic potassium cyanide then we obtain an \[\alpha -\] hydroxy ketone called benzoin as the product.

2) Knoevenagel Reaction
An example of this reaction is when benzaldehyde condenses with malonic acid in presence of pyridine as a basic catalyst. Here malonic acid and the base present generate a carbanion which acts as a nucleophile and attacks on the benzaldehyde.

Note: A condensation reaction is a closure of an organic addition reaction that typically proceeds in a step –wise fashion to produce an addition product, usually in equilibrium and a water molecule is released. In organic chemistry, nucleophilic substitution is a fundamental class of reaction in which a leaving group is replaced by an electron rich compound and the main involved is called substrate.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




