
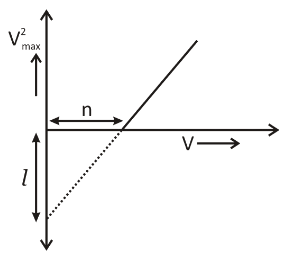
Give a brief description of the basic elementary process involved in the photoelectric emission in Einstein’s picture. When a photosensitive material is irradiated with the light of frequency \[v\]. The maximum the speed of electrons is given by\[{V_{\max }}\]. A plot of\[V_{\max }^2\]is found to vary with frequency\[v\]as shown in the figure. Use Einstein’s photoelectric equation to find the expression for
(a) Planck's constant
(b) Work function of the given photosensitive material in terms of the parameters \[l\] , \[n\] and mass \[m\] of the electron.

Answer
567k+ views
Hint: First of all, we will have to find the expression for the maximum kinetic energy. We know that an electron is not emitted if the frequency is less than the threshold frequency. In that case the kinetic energy is zero. There will be two velocities. We will have to manipulate accordingly and obtain the result.
Formula used:
Einstein explained the photoelectric effect on the basis of quantum theory. As we know the formula of work function, \[h{v_0} = \dfrac{{hc}}{{{\lambda _0}}}\]
Here c is the speed of light, h is the Planck’s constant and \[\nu \] is the frequency.
By substituting all the values in the given formula, we can find the value of Planck’s constant and the work function, W. If we define the work-function of a metal, it is the minimum energy required to eject one electron from metal.
Complete step by step solution:
(a) Let\[v\] be the frequency,\[hv\] be the energy of the proton,\[W\] be a work function and \[{E_K}\]. Be the maximum kinetic energy of the photoelectron.
\[hv = W + {E_K}\]
\[\Rightarrow{E_K} = h{v_0} - W\] …… (A)
Let \[{v_0}\]be threshold frequency, no electron will be emitted if frequency of incident light is less than the threshold frequency.
\[0 = h{v_0} - W\]
Let \[{\lambda _0}\] be the threshold wavelength.
Then, \[{v_o} = \dfrac{c}{{{\lambda _0}}}\]
Work function
\[W = h{v_0} \\
\Rightarrow W= \dfrac{{hc}}{{{\lambda _0}}}\] …… (B)
Where \[c\] is the speed of light.
Substitute the value in equation (A)
\[{E_{_K}} - hv = h{v_0}\]
\[\Rightarrow\dfrac{1}{2}m{v^2} = hv - h{v_0}\]
(b) let \[v_1^2\] and \[v_2^2\] be the velocities of the emitted electrons
\[h{v_1} = hv + \dfrac{1}{2}mv_1^2\] …… (C)
\[\Rightarrow h{v_2} = hv + \dfrac{1}{2}mv_2^2\] …… (D)
From equation (C) and (D)
\[h\left( {{v_2} - {v_1}} \right)\]=\[\dfrac{1}{2}m\left( {v_2^2 - v_1^2} \right)\]
\[\Rightarrow h = \dfrac{{\dfrac{1}{2}m\left( {v_2^2 - v_1^2} \right)}}{{\left( {{v_2} - {v_1}} \right)}}\]
Slope of \[v_{\max }^2\] vs frequency graph is:
\[\tan \theta = \dfrac{{v_2^2 - v_1^2}}{{{v_2} - {v_1}}}\]
\[\Rightarrow h = \dfrac{1}{2}m.\tan \theta \]
From graph we have
\[\tan \theta = \dfrac{1}{n}\]
So, \[h = \dfrac{1}{2}m\left( {\dfrac{1}{n}} \right)\] …… (E)
Again, for the work function of the material is (from the graph):
\[w = hn\] …… (F)
From the (E) and (F), we have:
\[
w = \dfrac{1}{2}m\left( {\dfrac{1}{n}} \right)n \\
\therefore w = \dfrac{1}{2}ml \\
\]
Note: The photoelectric effect is the emission of electrons when electromagnetic radiation, such as light, hits a material. Electrons emitted in this manner are called photoelectrons. Since light is bundled up into photons, Einstein concluded that when a photon falls on the surface of a metal, the entire photon's energy is transferred to the electron. Some energy is used to remove the electron from the metal atom and rest energy is given to the ejected electron. This photoelectric effect helps us to understand the quantum nature of light.
Formula used:
Einstein explained the photoelectric effect on the basis of quantum theory. As we know the formula of work function, \[h{v_0} = \dfrac{{hc}}{{{\lambda _0}}}\]
Here c is the speed of light, h is the Planck’s constant and \[\nu \] is the frequency.
By substituting all the values in the given formula, we can find the value of Planck’s constant and the work function, W. If we define the work-function of a metal, it is the minimum energy required to eject one electron from metal.
Complete step by step solution:
(a) Let\[v\] be the frequency,\[hv\] be the energy of the proton,\[W\] be a work function and \[{E_K}\]. Be the maximum kinetic energy of the photoelectron.
\[hv = W + {E_K}\]
\[\Rightarrow{E_K} = h{v_0} - W\] …… (A)
Let \[{v_0}\]be threshold frequency, no electron will be emitted if frequency of incident light is less than the threshold frequency.
\[0 = h{v_0} - W\]
Let \[{\lambda _0}\] be the threshold wavelength.
Then, \[{v_o} = \dfrac{c}{{{\lambda _0}}}\]
Work function
\[W = h{v_0} \\
\Rightarrow W= \dfrac{{hc}}{{{\lambda _0}}}\] …… (B)
Where \[c\] is the speed of light.
Substitute the value in equation (A)
\[{E_{_K}} - hv = h{v_0}\]
\[\Rightarrow\dfrac{1}{2}m{v^2} = hv - h{v_0}\]
(b) let \[v_1^2\] and \[v_2^2\] be the velocities of the emitted electrons
\[h{v_1} = hv + \dfrac{1}{2}mv_1^2\] …… (C)
\[\Rightarrow h{v_2} = hv + \dfrac{1}{2}mv_2^2\] …… (D)
From equation (C) and (D)
\[h\left( {{v_2} - {v_1}} \right)\]=\[\dfrac{1}{2}m\left( {v_2^2 - v_1^2} \right)\]
\[\Rightarrow h = \dfrac{{\dfrac{1}{2}m\left( {v_2^2 - v_1^2} \right)}}{{\left( {{v_2} - {v_1}} \right)}}\]
Slope of \[v_{\max }^2\] vs frequency graph is:
\[\tan \theta = \dfrac{{v_2^2 - v_1^2}}{{{v_2} - {v_1}}}\]
\[\Rightarrow h = \dfrac{1}{2}m.\tan \theta \]
From graph we have
\[\tan \theta = \dfrac{1}{n}\]
So, \[h = \dfrac{1}{2}m\left( {\dfrac{1}{n}} \right)\] …… (E)
Again, for the work function of the material is (from the graph):
\[w = hn\] …… (F)
From the (E) and (F), we have:
\[
w = \dfrac{1}{2}m\left( {\dfrac{1}{n}} \right)n \\
\therefore w = \dfrac{1}{2}ml \\
\]
Note: The photoelectric effect is the emission of electrons when electromagnetic radiation, such as light, hits a material. Electrons emitted in this manner are called photoelectrons. Since light is bundled up into photons, Einstein concluded that when a photon falls on the surface of a metal, the entire photon's energy is transferred to the electron. Some energy is used to remove the electron from the metal atom and rest energy is given to the ejected electron. This photoelectric effect helps us to understand the quantum nature of light.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




