
Formula of soap:
(A)- ${{C}_{16}}{{H}_{32}}COONa$
(B)- ${{C}_{17}}{{H}_{35}}COONa$
(C)- ${{C}_{17}}{{H}_{33}}COONa$
(D)- ${{C}_{17}}{{H}_{36}}COONa$
Answer
589.5k+ views
Hint: Soaps are sodium or potassium salts of long chain fatty acids. Fatty acids are carboxylic acids containing long saturated or unsaturated aliphatic chains containing 12-18 carbon atoms. Soap is represented as $RCO{{O}^{-}}N{{a}^{+}}$, where R is a long carbon chain.
Complete step by step solution:
Fats and oils are the triesters of glycerol commonly known as triglycerides. Soap is prepared by the hydrolysis of triglycerides with alkalies, and this process of preparation of soap by alkaline hydrolysis of fats and oils is called saponification.
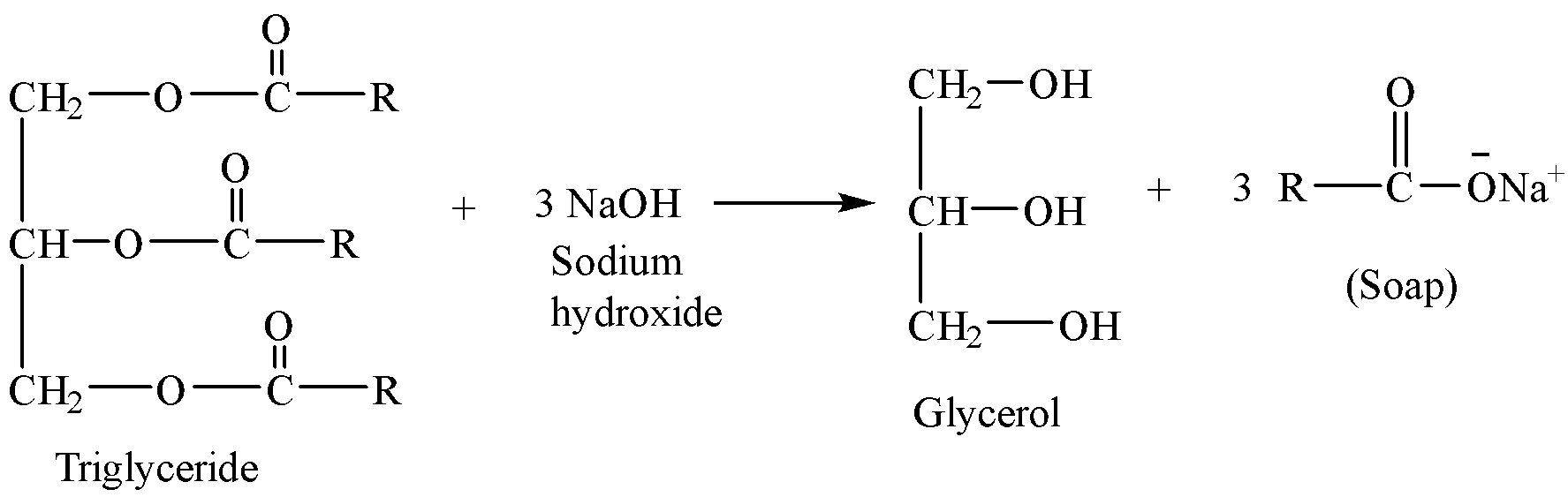
Sodium salts of some fatty acids which are most commonly used as soap are stearic acid (${{C}_{17}}{{H}_{35}}COOH$), oleic acid (${{C}_{17}}{{H}_{33}}COOH$) and palmitic acid (${{C}_{15}}{{H}_{31}}COOH$).
In general, we can write a formula for salts of saturated fatty acids as ${{C}_{n}}{{H}_{2n+1}}COONa$ and those of mono-unsaturated (containing only one double bond) fatty acids as ${{C}_{n}}{{H}_{2n-1}}COONa$.
Now let us examine all the options to find the correct formula representing soap.
${{C}_{16}}{{H}_{32}}COONa$ and ${{C}_{17}}{{H}_{36}}COONa$
These compounds do not fit the formulas of either saturated or unsaturated salts of carboxylic acid.
${{C}_{17}}{{H}_{35}}COONa$
This is sodium salt of stearic acid and widely used as soap.
${{C}_{17}}{{H}_{33}}COONa$
It is sodium salt of oleic acid. Oleic acid (${{C}_{17}}{{H}_{33}}COOH$) is an unsaturated fatty acid and contains one double bond at ${{C}_{9}}$ in cis-orientation.
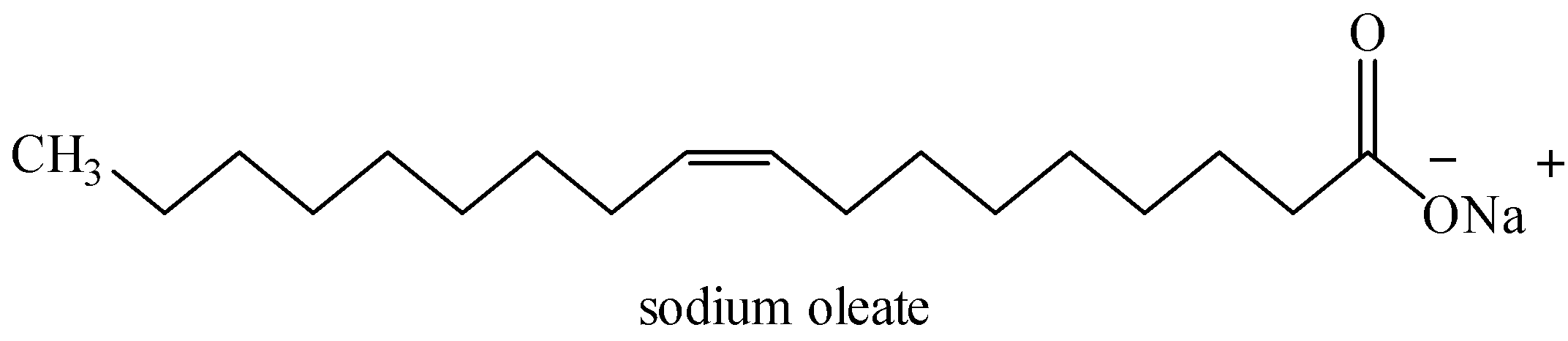
As we already know that soaps are sodium salts of stearic or oleic acids. Therefore, the formula for soap can be ${{C}_{17}}{{H}_{35}}COONa$ and ${{C}_{17}}{{H}_{33}}COONa$.
Now, two of the options can be correct, i.e. (B) and (C). But since sodium stearate is generally referred to as soap.
Hence, the correct option will be (B).
Note: Note that both sodium stearate (${{C}_{17}}{{H}_{35}}COONa$) and sodium oleate (${{C}_{17}}{{H}_{33}}COONa$) are used as the major component of soap. But because ${{C}_{17}}{{H}_{35}}COONa$ is most commonly used in bar soaps, so we choose ${{C}_{17}}{{H}_{35}}COONa$ as the formula of soap between ${{C}_{17}}{{H}_{35}}COONa$ and ${{C}_{17}}{{H}_{33}}COONa$.
Complete step by step solution:
Fats and oils are the triesters of glycerol commonly known as triglycerides. Soap is prepared by the hydrolysis of triglycerides with alkalies, and this process of preparation of soap by alkaline hydrolysis of fats and oils is called saponification.

Sodium salts of some fatty acids which are most commonly used as soap are stearic acid (${{C}_{17}}{{H}_{35}}COOH$), oleic acid (${{C}_{17}}{{H}_{33}}COOH$) and palmitic acid (${{C}_{15}}{{H}_{31}}COOH$).
In general, we can write a formula for salts of saturated fatty acids as ${{C}_{n}}{{H}_{2n+1}}COONa$ and those of mono-unsaturated (containing only one double bond) fatty acids as ${{C}_{n}}{{H}_{2n-1}}COONa$.
Now let us examine all the options to find the correct formula representing soap.
${{C}_{16}}{{H}_{32}}COONa$ and ${{C}_{17}}{{H}_{36}}COONa$
These compounds do not fit the formulas of either saturated or unsaturated salts of carboxylic acid.
${{C}_{17}}{{H}_{35}}COONa$
This is sodium salt of stearic acid and widely used as soap.
${{C}_{17}}{{H}_{33}}COONa$
It is sodium salt of oleic acid. Oleic acid (${{C}_{17}}{{H}_{33}}COOH$) is an unsaturated fatty acid and contains one double bond at ${{C}_{9}}$ in cis-orientation.

As we already know that soaps are sodium salts of stearic or oleic acids. Therefore, the formula for soap can be ${{C}_{17}}{{H}_{35}}COONa$ and ${{C}_{17}}{{H}_{33}}COONa$.
Now, two of the options can be correct, i.e. (B) and (C). But since sodium stearate is generally referred to as soap.
Hence, the correct option will be (B).
Note: Note that both sodium stearate (${{C}_{17}}{{H}_{35}}COONa$) and sodium oleate (${{C}_{17}}{{H}_{33}}COONa$) are used as the major component of soap. But because ${{C}_{17}}{{H}_{35}}COONa$ is most commonly used in bar soaps, so we choose ${{C}_{17}}{{H}_{35}}COONa$ as the formula of soap between ${{C}_{17}}{{H}_{35}}COONa$ and ${{C}_{17}}{{H}_{33}}COONa$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




