
Formula for ferrocene is
A.\[{\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{4 - }}}}\]
B.\[{\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 + }}}}\]
C.\[\left[ {{\text{Fe(CO}}{{\text{)}}_{\text{5}}}} \right]\]
D.\[\left[ {{\text{Fe(}}{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{{\text{)}}_{\text{2}}}} \right]\]
Answer
564.9k+ views
Hint:Ferrocene is actually a metallocene or we can say the first sandwich compound. Ferrocenes are the molecules where metal atoms are sandwiched between two parallel and planar cyclopentadienyl rings. Organometallic compound of \[{\text{Fe}}\] is ferrocene.
Complete step by step answer:
In ferrocene \[{\text{Fe}}\] metal is attached with a ligand called cyclopentadiene, the formula for cyclopentadiene is \[{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}\]. In ferrocene, two cyclopentadienyl ligand present so the formula is \[\left[ {{\text{Fe(}}{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{{\text{)}}_{\text{2}}}} \right]\]. Two important conformers of ferrocene are: -
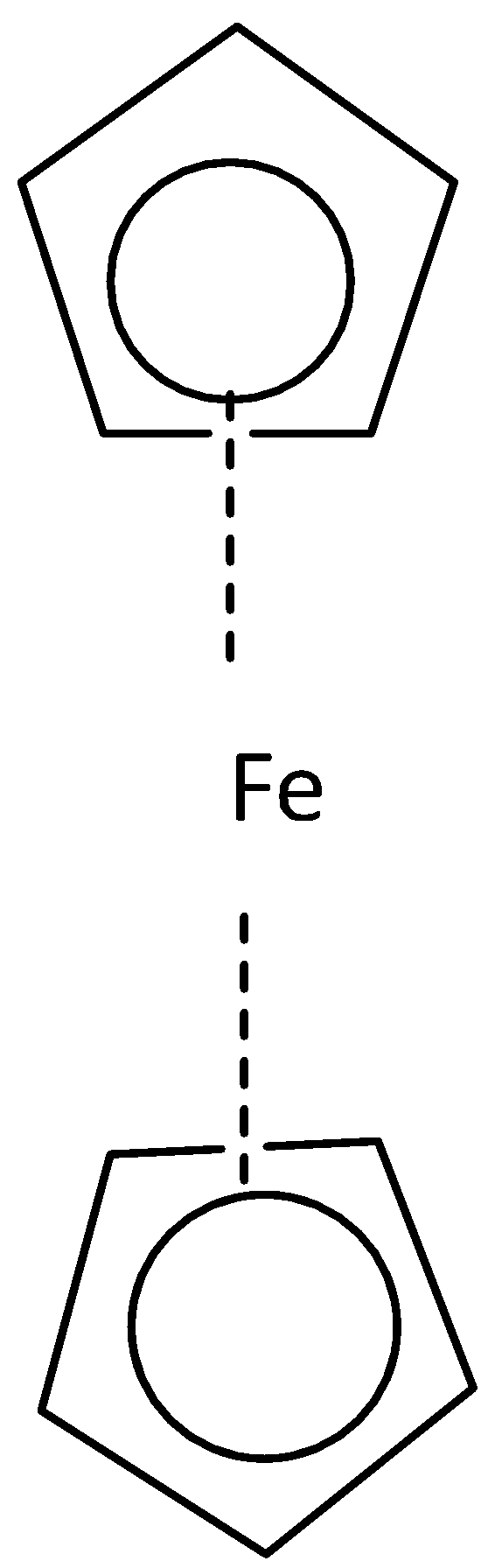
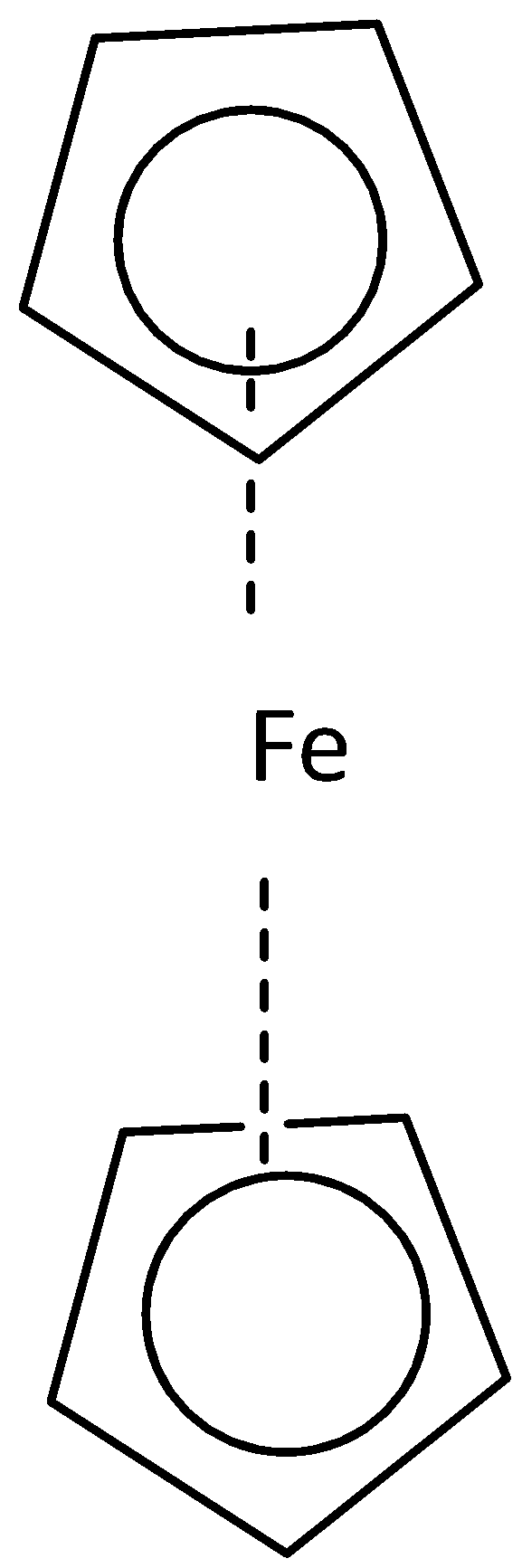
Where both cyclopentadienyl rings are in the opposite direction that is the staggered conformation of ferrocene on the other hand where both the cyclopentadienyl rings in the same direction that is the eclipsed conformation. Point group for staggered conformation of ferrocene is \[{{\text{D}}_{{\text{5d}}}}\] and for eclipsed conformation is \[{{\text{D}}_{{\text{5h}}}}\].
Therefore we can conclude that option D is the correct option, that is \[\left[ {{\text{Fe(}}{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{{\text{)}}_{\text{2}}}} \right]\]
Additional information:
Ferrocene undergoes electrophilic substitution reaction faster than benzene indicating that electrons of Cp ring are more readily available. Some electrophilic reactions of ferrocene are given ahead:
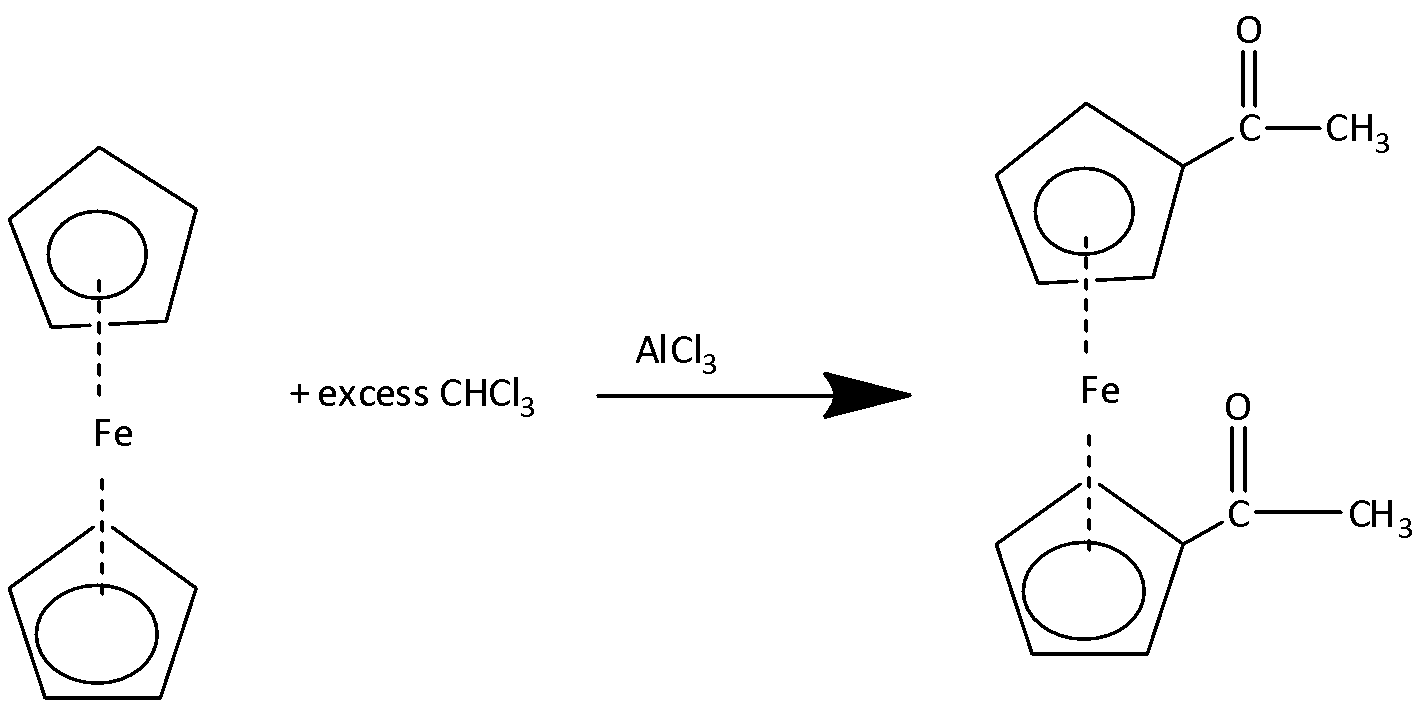
1.Friedel-Craft Acylation:
The reaction of ferrocene with acetyl chloride in the presence of \[{\text{AlC}}{{\text{l}}_{\text{3}}}\] produces mono acetyl derivative of ferrocene.

If the reaction is carried out in the presence of excess amounts of acetyl chloride then diacetyl derivatives of ferrocene are formed.
2,Friedel-Craft Alkylation:
In this reaction we used alkyl halide and \[{\text{AlC}}{{\text{l}}_{\text{3}}}\]at \[{\text{200 atm}}\]to produced mono alkyl derivative of ferrocene. If this reaction is carried out in the presence of excess amounts of alkyl chloride then the dialkyl derivative will be formed.

Note: The cyclopentadiene rings of metallocene are aromatic in nature therefore they do not undergo conjugate diene such as Diels alder reaction. They undergo electrophilic substitution reactions which are characteristic of aromatic compounds.
Complete step by step answer:
In ferrocene \[{\text{Fe}}\] metal is attached with a ligand called cyclopentadiene, the formula for cyclopentadiene is \[{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}\]. In ferrocene, two cyclopentadienyl ligand present so the formula is \[\left[ {{\text{Fe(}}{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{{\text{)}}_{\text{2}}}} \right]\]. Two important conformers of ferrocene are: -


- (a) Staggered conformation (b) eclipsed conformation
Where both cyclopentadienyl rings are in the opposite direction that is the staggered conformation of ferrocene on the other hand where both the cyclopentadienyl rings in the same direction that is the eclipsed conformation. Point group for staggered conformation of ferrocene is \[{{\text{D}}_{{\text{5d}}}}\] and for eclipsed conformation is \[{{\text{D}}_{{\text{5h}}}}\].
Therefore we can conclude that option D is the correct option, that is \[\left[ {{\text{Fe(}}{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{5}}}{{\text{)}}_{\text{2}}}} \right]\]
Additional information:
Ferrocene undergoes electrophilic substitution reaction faster than benzene indicating that electrons of Cp ring are more readily available. Some electrophilic reactions of ferrocene are given ahead:
1.Friedel-Craft Acylation:
The reaction of ferrocene with acetyl chloride in the presence of \[{\text{AlC}}{{\text{l}}_{\text{3}}}\] produces mono acetyl derivative of ferrocene.

If the reaction is carried out in the presence of excess amounts of acetyl chloride then diacetyl derivatives of ferrocene are formed.

2,Friedel-Craft Alkylation:
In this reaction we used alkyl halide and \[{\text{AlC}}{{\text{l}}_{\text{3}}}\]at \[{\text{200 atm}}\]to produced mono alkyl derivative of ferrocene. If this reaction is carried out in the presence of excess amounts of alkyl chloride then the dialkyl derivative will be formed.

Note: The cyclopentadiene rings of metallocene are aromatic in nature therefore they do not undergo conjugate diene such as Diels alder reaction. They undergo electrophilic substitution reactions which are characteristic of aromatic compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




