
For the molecule of $I{{F}_{7}}$.
(a)State the hybridisation of the central atom.
(b)State the geometry of the molecule.
Answer
592.5k+ views
Hint: Hybridization of a molecule is determined using the postulates of the Valence band theory and its geometry can be formulated by using the VSEPR rule. The geometry depends on the no. of lone pairs and bond pairs.
Complete step by step answer:
(a)Hybridization is when the concept of atomic orbitals fuse to make new hybridized orbitals, which successively influences molecular geometry and bonding properties. Hybridization is also an expansion of the valence bond theory.
According to Valence band theory the metal atom or ion in the influence of ligands uses its (n-1)d, ns, np, or ns, np, nd orbitals for hybridization to yield a group of equivalent orbitals of definite geometry like octahedral, tetrahedral, square planar then on.(Here ligands refers to ion or molecule, which donates a pair of electron to the central metal These hybrid orbitals are allowed to overlap with ligand orbitals which will donate electron pairs for bonding.
Hybridisation = Number of valence electrons on central atom + Number of monovalent surrounding atoms (like hydrogen and halogen)- positive charge (if on complex) + anionic charge (if on complex) and divide this whole by 2.
Now, for $I{{F}_{7}}$: Hybridisation = 7 (number of valence electrons on I-atom) + 7 (number of surrounding halogen atoms) -0+0 and divide this whole by 2. The element Iodine has 7 lone electrons. When it is bonded to seven Fluorine atoms through sigma bonds, the total number of electron pairs around Iodine becomes 7 with no lone pair of el
(Ground State)

(Excited State)

$s{{p}^{3}}{{d}^{3}}$- Hybridization ($I{{F}_{7}}$)
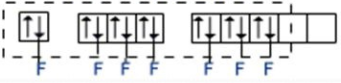
(Ground State)
(Excited State)
$s{{p}^{3}}{{d}^{3}}$- Hybridization ($I{{F}_{7}}$)
Thus, the hybridisation of $I{{F}_{7}}$is $s{{p}^{3}}{{d}^{3}}$ with no lone pair of electrons and this corresponds to pentagonal bipyramidal geometry.
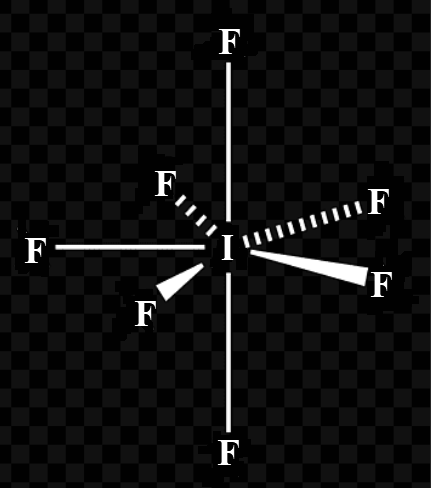
(b) Iodine heptafluoride, $I{{F}_{7}}$, may be an example of a pentagonal bipyramidal geometry. VSEPR rules suggest seven electron pairs. These are made up from six bonding pairs and one lone pair.
Note:
-VSEPR theory has certain postulates which one must follow to deduce the geometrical structure of a compound.
-It can be noted that sigma bonds involve the head-to-head overlapping of atomic orbitals whereas pi bonds involve parallel overlapping.
Complete step by step answer:
(a)Hybridization is when the concept of atomic orbitals fuse to make new hybridized orbitals, which successively influences molecular geometry and bonding properties. Hybridization is also an expansion of the valence bond theory.
According to Valence band theory the metal atom or ion in the influence of ligands uses its (n-1)d, ns, np, or ns, np, nd orbitals for hybridization to yield a group of equivalent orbitals of definite geometry like octahedral, tetrahedral, square planar then on.(Here ligands refers to ion or molecule, which donates a pair of electron to the central metal These hybrid orbitals are allowed to overlap with ligand orbitals which will donate electron pairs for bonding.
Hybridisation = Number of valence electrons on central atom + Number of monovalent surrounding atoms (like hydrogen and halogen)- positive charge (if on complex) + anionic charge (if on complex) and divide this whole by 2.
Now, for $I{{F}_{7}}$: Hybridisation = 7 (number of valence electrons on I-atom) + 7 (number of surrounding halogen atoms) -0+0 and divide this whole by 2. The element Iodine has 7 lone electrons. When it is bonded to seven Fluorine atoms through sigma bonds, the total number of electron pairs around Iodine becomes 7 with no lone pair of el
(Ground State)

(Excited State)

$s{{p}^{3}}{{d}^{3}}$- Hybridization ($I{{F}_{7}}$)

(Ground State)
(Excited State)
$s{{p}^{3}}{{d}^{3}}$- Hybridization ($I{{F}_{7}}$)
Thus, the hybridisation of $I{{F}_{7}}$is $s{{p}^{3}}{{d}^{3}}$ with no lone pair of electrons and this corresponds to pentagonal bipyramidal geometry.

(b) Iodine heptafluoride, $I{{F}_{7}}$, may be an example of a pentagonal bipyramidal geometry. VSEPR rules suggest seven electron pairs. These are made up from six bonding pairs and one lone pair.
Note:
-VSEPR theory has certain postulates which one must follow to deduce the geometrical structure of a compound.
-It can be noted that sigma bonds involve the head-to-head overlapping of atomic orbitals whereas pi bonds involve parallel overlapping.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




