
For the identification of beta $\beta $-naphthol using dye test, it is necessary to use:
A. dichloromethane solution of $\beta $-naphthol
B. acidic solution of $\beta $-naphthol
C. neutral solution of$\beta $-naphthol
D. alkaline solution of $\beta $-naphthol
Answer
591.9k+ views
Hint: We know that azo dye test is used for identification of aromatic primary amines. Primary amine is the amine which is in the form of ${\rm{R}} - {\rm{N}}{{\rm{H}}_{\rm{2}}}$ (R is any alkyl group).
Complete step by step answer:
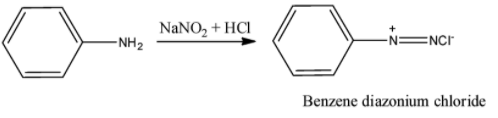
The aromatic primary amine can be confirmed by azo dye test. In this test, primary aromatic amine like aniline reacts with nitrous acid (produced by reaction of sodium nitrite with hydrochloric acid) at zero to five degree Celsius to form diazonium salt or benzene diazonium chloride. The reaction of formed benzene diazonium chloride with $\beta $-naphthol gives a scarlet red dye and this die is sparingly soluble in water. The reaction can be shown as follows:
Let’s come to the question. We have to identify the type of solution of $\beta $-naphthol to be used in dye test. If the solution of $\beta $-naphthol is alkaline, -OH convert to ${{\rm{O}}^ - }$ which reacts with azo compound (benzene diazonium chloride) to form azo dye. The chemical reaction is shown below.
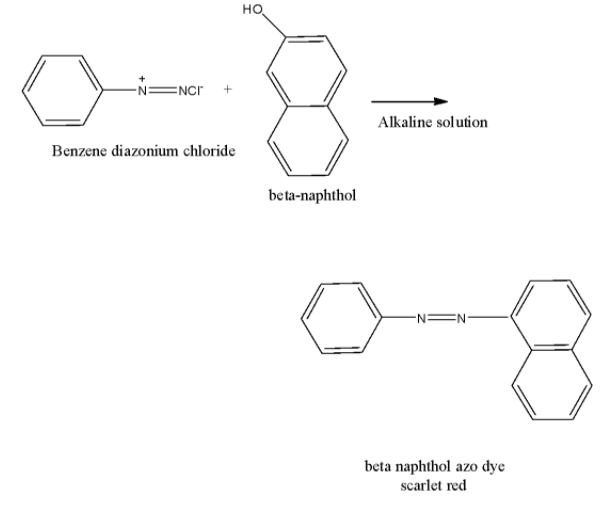
Hence, the correct option is D, that is, an alkaline solution.
Additional Information Another test of amine is carbylamine test. In this test aromatic as well as aliphatic primary amine is heated with chloroform to result in carbylamine. The reaction is,
${\rm{R}} - {\rm{N}}{{\rm{H}}_{\rm{2}}} + {\rm{CHC}}{{\rm{l}}_{\rm{3}}} + 3{\rm{KOH}} \to {\rm{RNC}}\left( {{\rm{Carbylamine}}} \right) + 3{\rm{KCl + 3}}{{\rm{H}}_2}{\rm{O}}$
Note: It is to be noted that aromatic primary can be distinguished from secondary and tertiary amine by azo dye test and aliphatic or aromatic primary amine can be differentiated from secondary and tertiary amine by Carbylamine test.
Complete step by step answer:
The aromatic primary amine can be confirmed by azo dye test. In this test, primary aromatic amine like aniline reacts with nitrous acid (produced by reaction of sodium nitrite with hydrochloric acid) at zero to five degree Celsius to form diazonium salt or benzene diazonium chloride. The reaction of formed benzene diazonium chloride with $\beta $-naphthol gives a scarlet red dye and this die is sparingly soluble in water. The reaction can be shown as follows:

Let’s come to the question. We have to identify the type of solution of $\beta $-naphthol to be used in dye test. If the solution of $\beta $-naphthol is alkaline, -OH convert to ${{\rm{O}}^ - }$ which reacts with azo compound (benzene diazonium chloride) to form azo dye. The chemical reaction is shown below.

Hence, the correct option is D, that is, an alkaline solution.
Additional Information Another test of amine is carbylamine test. In this test aromatic as well as aliphatic primary amine is heated with chloroform to result in carbylamine. The reaction is,
${\rm{R}} - {\rm{N}}{{\rm{H}}_{\rm{2}}} + {\rm{CHC}}{{\rm{l}}_{\rm{3}}} + 3{\rm{KOH}} \to {\rm{RNC}}\left( {{\rm{Carbylamine}}} \right) + 3{\rm{KCl + 3}}{{\rm{H}}_2}{\rm{O}}$
Note: It is to be noted that aromatic primary can be distinguished from secondary and tertiary amine by azo dye test and aliphatic or aromatic primary amine can be differentiated from secondary and tertiary amine by Carbylamine test.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




