
How many following oxyacids have peroxy linkage?
${H_2}S{O_3}$,${H_2}{S_2}{O_8}$,${H_3}P{O_2}$,${H_3}P{O_5}$,$HCl{O_2}$
Answer
559.2k+ views
Hint: Oxyacids are acidic substances in which one or more hydrogen atoms are bonded to oxygen atoms which are further bonded to other elements subsequently. The compound containing the o-o bond has peroxy linkage.
Complete step by step answer:
We know that oxyacid is known as oxygen containing acid. Oxyacids are prepared by reacting von-metallic acids with water i.e. they react with water to form oxyacids that yield hydronium $\left( {{H_3}{O^ + }} \right)$ in solution. The strength of an oxyacid is the determinacy extent to which it dissociates in water. The acid strength can be predicted on the basis of oxidation number and oxidation number. More is electronegativity of an element; more will be its acidic character.
Moreover, as we know prosy linkage is defined as the peroxide linkage or is simply $o - o$. The single bond present between two oxygen atoms is known as proxy linkage.
Now let us find which of the given compounds has proxy linkage.
(1). ${H_2}S{O_3}$ This compound is commonly known as sulfurous acid. This hybridization of $S$ is $s{p^3}$ and its structure is tetrahedral.
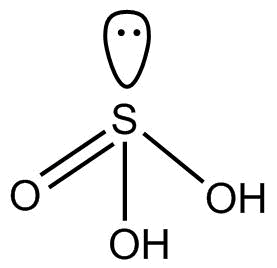
So we can see that there is no proxy linkage in ${H_2}S{O_3}$.
(2).${H_2}{S_2}{O_8}$ This compound is chemically known as peroxydisulfuric acid. The hybridization of each $S$ is $s{p^3}$ and its structure is as below:-
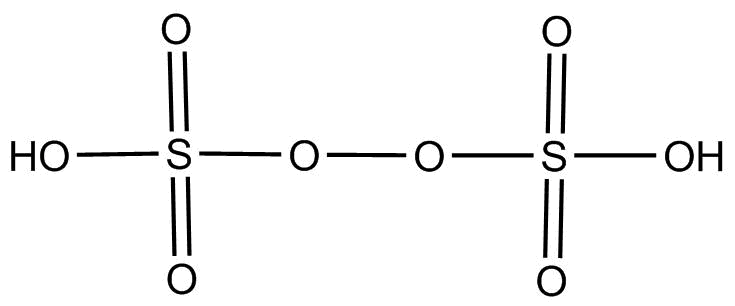
Since $o - o$ linkage is present is its structure. So this compound contains proxy linkage.
(3). ${H_3}P{O_2}$ This compound is known as hypophosphorous acid and the hybridization of $P$ is $s{p^3}$ and its structure is:
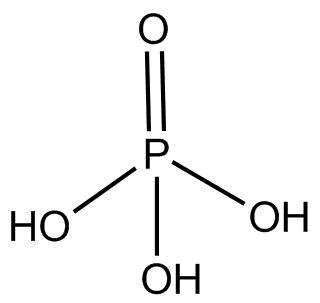
There is no proxy linkage present in this compound.
(4).${H_3}P{O_5}$ This compound is known as peroxymonophosplatic acid and its structure is
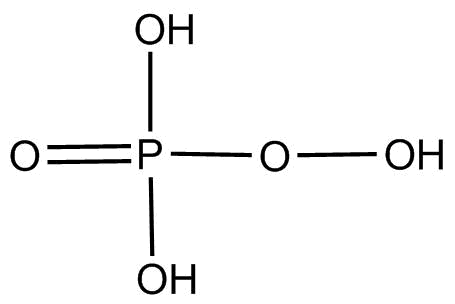
We can clearly see $o - o$ linkage present in the compound. Thus it is oxyacid with proxy linkage.
(5). $HCl$${O_2}$ This compound is known as chorus acid and the hybridization of $cl$atom in the compound is $s{p^3}$.it is structure is as below
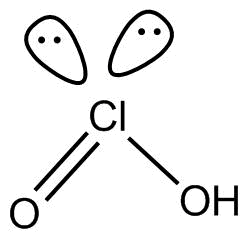
No proxy linkage is present in the compound.
Thus on our observation, we got to know that ${H_2}{S_2}{O_8}$ and ${H_3}P{O_5}$ contains proxy linkage.
Note:
The strength of an oxyacid is defined by the extent to which it dissociates in water [i.e. ability to form ${H^ + }$ions]. Oxyacids are mainly used in the synthesis of other chemical compounds and have a wide range of industrial applications.
Complete step by step answer:
We know that oxyacid is known as oxygen containing acid. Oxyacids are prepared by reacting von-metallic acids with water i.e. they react with water to form oxyacids that yield hydronium $\left( {{H_3}{O^ + }} \right)$ in solution. The strength of an oxyacid is the determinacy extent to which it dissociates in water. The acid strength can be predicted on the basis of oxidation number and oxidation number. More is electronegativity of an element; more will be its acidic character.
Moreover, as we know prosy linkage is defined as the peroxide linkage or is simply $o - o$. The single bond present between two oxygen atoms is known as proxy linkage.
Now let us find which of the given compounds has proxy linkage.
(1). ${H_2}S{O_3}$ This compound is commonly known as sulfurous acid. This hybridization of $S$ is $s{p^3}$ and its structure is tetrahedral.

So we can see that there is no proxy linkage in ${H_2}S{O_3}$.
(2).${H_2}{S_2}{O_8}$ This compound is chemically known as peroxydisulfuric acid. The hybridization of each $S$ is $s{p^3}$ and its structure is as below:-

Since $o - o$ linkage is present is its structure. So this compound contains proxy linkage.
(3). ${H_3}P{O_2}$ This compound is known as hypophosphorous acid and the hybridization of $P$ is $s{p^3}$ and its structure is:

There is no proxy linkage present in this compound.
(4).${H_3}P{O_5}$ This compound is known as peroxymonophosplatic acid and its structure is

We can clearly see $o - o$ linkage present in the compound. Thus it is oxyacid with proxy linkage.
(5). $HCl$${O_2}$ This compound is known as chorus acid and the hybridization of $cl$atom in the compound is $s{p^3}$.it is structure is as below

No proxy linkage is present in the compound.
Thus on our observation, we got to know that ${H_2}{S_2}{O_8}$ and ${H_3}P{O_5}$ contains proxy linkage.
Note:
The strength of an oxyacid is defined by the extent to which it dissociates in water [i.e. ability to form ${H^ + }$ions]. Oxyacids are mainly used in the synthesis of other chemical compounds and have a wide range of industrial applications.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




