
Following are the transition metal ions of 3d series:
${\text{T}}{{\text{i}}^{{\text{4 + }}}}{\text{,}}\,{{\text{V}}^{{\text{2 + }}}}{\text{,}}\,{\text{M}}{{\text{n}}^{{\text{3 + }}}}{\text{,}}\,{\text{C}}{{\text{r}}^{{\text{3 + }}}}$
(Atomic number: Ti=22, V= 23, Cr-24,Mn=25)
Answer the following:
(i) Which ion is most stable in an aqueous solution and why?
(ii) Which ion is a strong oxidizing agent and why?
(iii) Which ion is colourless and why?
Answer
578.4k+ views
Hint: Most stable ion is an ion that has a full-filled or half-filled electronic configuration. The species having high reduction potential will be the strongest oxidizing agent. The ion having no electron in orbitals will be colourless.
Complete step by step answer:
(i) We can determine the most stable ion in an aqueous solution as follows:
Each metal reacts with water ligands and forms an octahedral complex as follows:
${{\text{M}}^{{\text{n + }}}}\,{\text{ + }}\,{\text{6}}\,{{\text{H}}_{\text{2}}}{\text{O}}\, \to \,{\left[ {{\text{M}}{{\left( {{{\text{H}}_{\text{2}}}{\text{O}}} \right)}_{\text{6}}}} \right]^{{\text{n + }}}}$
As the water ligand approaches metal, the d-orbitals of metal split into two parts, three orbitals of lower energy designated as ${t_{2g}}$ and two orbitals of higher energy designated as ${e_g}$.
The valence electrons of the metal get rearranged in these two set of orbitals. The water is a weak field ligand, so it does not cause pairing. The full-filled and half-filled electronic configuration are most stable thus stabilizing the metal ion.
The valence electronic configuration of each metal ion, its crystal field splitting and filling of electrons is shown as follows:
Valence electronic configuration of ${\text{T}}{{\text{i}}^{4 + }}\, = \,3{{\text{d}}^0}$
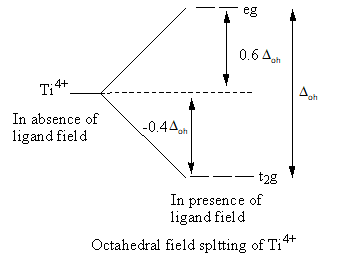
So, the electronic configuration of the titanium metal on forming the complex is $t_{2g}^0$ .
Valence electronic configuration of ${{\text{V}}^{2 + }}\, = \,3{{\text{d}}^3}$
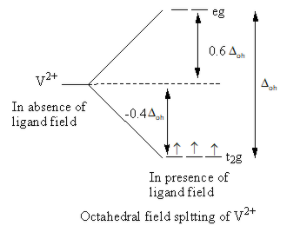
So, the electronic configuration of the vanadium metal on forming the complex is $t_{2g}^3$ .
Valence electronic configuration of ${\text{C}}{{\text{r}}^{3 + }}\, = \,3{{\text{d}}^3}$
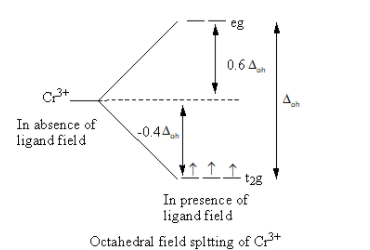
So, the electronic configuration of the chromium metal on forming the complex is $t_{2g}^3$ .
Valence electronic configuration of ${\text{M}}{{\text{n}}^{3 + }}\, = \,3{{\text{d}}^4}$
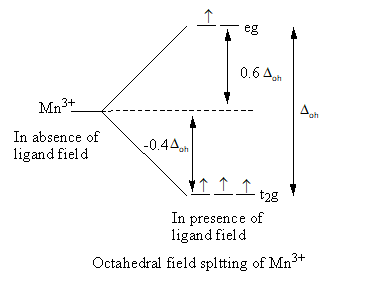
So, the electronic configuration of the manganese metal on forming the complex is $t_{2g}^3e_g^1$ .
So, vanadium ${{\text{V}}^{{\text{2 + }}}}$ and chromium metal ion${\text{C}}{{\text{r}}^{{\text{3 + }}}}$ have stable half-filled electronic configuration ${\text{T}}{{\text{i}}^{{\text{4 + }}}}{\text{,}}\,{{\text{V}}^{{\text{2 + }}}}{\text{,}}\,{\text{M}}{{\text{n}}^{{\text{3 + }}}}{\text{,}}\,{\text{C}}{{\text{r}}^{{\text{3 + }}}}$
Therefore, vanadium ${{\text{V}}^{{\text{2 + }}}}$ and chromium metal ion ${\text{C}}{{\text{r}}^{{\text{3 + }}}}$ are most stable in an aqueous solution due to stable half-filled electronic configuration.
(ii) Determine the strongest oxidizing agent as follows:
The reduction potential of ${\text{M}}{{\text{n}}^{{\text{3 + }}}}{\text{/M}}{{\text{n}}^{{\text{2 + }}}}$ is highest $4.45\,{\text{V}}$ , ${{\text{V}}^{{\text{3 + }}}}{\text{/}}{{\text{V}}^{{\text{2 + }}}}$ is $0.26\,V$, ${\text{C}}{{\text{r}}^{{\text{3 + }}}}{\text{/C}}{{\text{r}}^{{\text{2 + }}}}$ is $ - 0.41\,V$ and ${\text{T}}{{\text{i}}^{{\text{4 + }}}}{\text{/T}}{{\text{i}}^{{\text{3 + }}}}$ is highest $0.1\,{\text{V}}$.
So, the reduction potential of ${\text{M}}{{\text{n}}^{{\text{3 + }}}}$is highest so, will reduced easily and cause oxidation of another species so, it is the strongest oxidising agent among all four.
Therefore, ${\text{M}}{{\text{n}}^{{\text{3 + }}}}$ ion is a strong oxidizing agent due to high reduction potential.
(iii) Determine the colourless ion as follows:
The colour is produced by d-d transition means electron from one d-orbitals goes into another d-orbitals by releasing energy. The energy lies in the visible region, so a colour is observed. The metal ion ${\text{T}}{{\text{i}}^{{\text{4 + }}}}$ has no electron in orbitals so, d-d transition cannot take place so, the ${\text{T}}{{\text{i}}^{{\text{4 + }}}}$ is colourless.
Therefore, ${\text{T}}{{\text{i}}^{{\text{4 + }}}}$ ion is colourless due to absence of electrons in the metal orbitals in complex.
Note: The $t_{2g}^3$ is a more stable configuration then $t_{2g}^3e_g^1$. The presence of one electron in ${e_g}$ causes the John Teller distortion. High the reduction potential, stronger will be the oxidizing agent. The d-d transitions are responsible for colour in transition metals.
Complete step by step answer:
(i) We can determine the most stable ion in an aqueous solution as follows:
Each metal reacts with water ligands and forms an octahedral complex as follows:
${{\text{M}}^{{\text{n + }}}}\,{\text{ + }}\,{\text{6}}\,{{\text{H}}_{\text{2}}}{\text{O}}\, \to \,{\left[ {{\text{M}}{{\left( {{{\text{H}}_{\text{2}}}{\text{O}}} \right)}_{\text{6}}}} \right]^{{\text{n + }}}}$
As the water ligand approaches metal, the d-orbitals of metal split into two parts, three orbitals of lower energy designated as ${t_{2g}}$ and two orbitals of higher energy designated as ${e_g}$.
The valence electrons of the metal get rearranged in these two set of orbitals. The water is a weak field ligand, so it does not cause pairing. The full-filled and half-filled electronic configuration are most stable thus stabilizing the metal ion.
The valence electronic configuration of each metal ion, its crystal field splitting and filling of electrons is shown as follows:
Valence electronic configuration of ${\text{T}}{{\text{i}}^{4 + }}\, = \,3{{\text{d}}^0}$

So, the electronic configuration of the titanium metal on forming the complex is $t_{2g}^0$ .
Valence electronic configuration of ${{\text{V}}^{2 + }}\, = \,3{{\text{d}}^3}$

So, the electronic configuration of the vanadium metal on forming the complex is $t_{2g}^3$ .
Valence electronic configuration of ${\text{C}}{{\text{r}}^{3 + }}\, = \,3{{\text{d}}^3}$

So, the electronic configuration of the chromium metal on forming the complex is $t_{2g}^3$ .
Valence electronic configuration of ${\text{M}}{{\text{n}}^{3 + }}\, = \,3{{\text{d}}^4}$

So, the electronic configuration of the manganese metal on forming the complex is $t_{2g}^3e_g^1$ .
So, vanadium ${{\text{V}}^{{\text{2 + }}}}$ and chromium metal ion${\text{C}}{{\text{r}}^{{\text{3 + }}}}$ have stable half-filled electronic configuration ${\text{T}}{{\text{i}}^{{\text{4 + }}}}{\text{,}}\,{{\text{V}}^{{\text{2 + }}}}{\text{,}}\,{\text{M}}{{\text{n}}^{{\text{3 + }}}}{\text{,}}\,{\text{C}}{{\text{r}}^{{\text{3 + }}}}$
Therefore, vanadium ${{\text{V}}^{{\text{2 + }}}}$ and chromium metal ion ${\text{C}}{{\text{r}}^{{\text{3 + }}}}$ are most stable in an aqueous solution due to stable half-filled electronic configuration.
(ii) Determine the strongest oxidizing agent as follows:
The reduction potential of ${\text{M}}{{\text{n}}^{{\text{3 + }}}}{\text{/M}}{{\text{n}}^{{\text{2 + }}}}$ is highest $4.45\,{\text{V}}$ , ${{\text{V}}^{{\text{3 + }}}}{\text{/}}{{\text{V}}^{{\text{2 + }}}}$ is $0.26\,V$, ${\text{C}}{{\text{r}}^{{\text{3 + }}}}{\text{/C}}{{\text{r}}^{{\text{2 + }}}}$ is $ - 0.41\,V$ and ${\text{T}}{{\text{i}}^{{\text{4 + }}}}{\text{/T}}{{\text{i}}^{{\text{3 + }}}}$ is highest $0.1\,{\text{V}}$.
So, the reduction potential of ${\text{M}}{{\text{n}}^{{\text{3 + }}}}$is highest so, will reduced easily and cause oxidation of another species so, it is the strongest oxidising agent among all four.
Therefore, ${\text{M}}{{\text{n}}^{{\text{3 + }}}}$ ion is a strong oxidizing agent due to high reduction potential.
(iii) Determine the colourless ion as follows:
The colour is produced by d-d transition means electron from one d-orbitals goes into another d-orbitals by releasing energy. The energy lies in the visible region, so a colour is observed. The metal ion ${\text{T}}{{\text{i}}^{{\text{4 + }}}}$ has no electron in orbitals so, d-d transition cannot take place so, the ${\text{T}}{{\text{i}}^{{\text{4 + }}}}$ is colourless.
Therefore, ${\text{T}}{{\text{i}}^{{\text{4 + }}}}$ ion is colourless due to absence of electrons in the metal orbitals in complex.
Note: The $t_{2g}^3$ is a more stable configuration then $t_{2g}^3e_g^1$. The presence of one electron in ${e_g}$ causes the John Teller distortion. High the reduction potential, stronger will be the oxidizing agent. The d-d transitions are responsible for colour in transition metals.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




