
Find the Order of above Mechanism of Cannizzaro reaction of Benzaldehyde:
$
\left( A \right)1 \\
\left( B \right)2 \\
\left( C \right)3 \\
\left( D \right)4 \\
$

Answer
535.5k+ views
Hint: The order of reaction is the sum of coefficients of power of each reactant in rate law. It is the power dependence of the rate of concentration of each reactant. The formation of products requires two molecules of Aldehyde as well as one molecule of base to form a product. Hence the order represents the minimum requirement for formation of product.
Complete step by step solution:
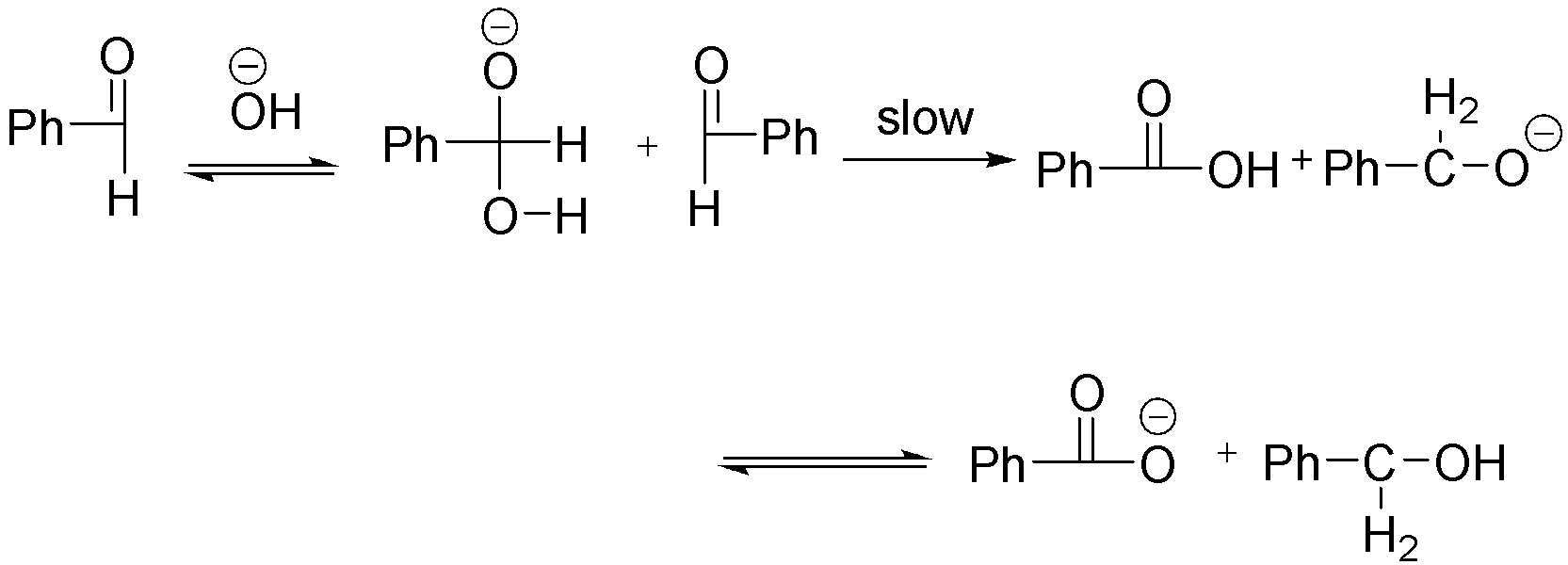
The better insight into the mechanism of reaction can be obtained with study of kinetics. The Cannizzaro reaction is an example of a Disproportionation reaction in organic chemistry. In this reaction a non-enolizable aldehyde can be Oxidized as well as reduced simultaneously. An aldehyde is oxidised to carboxylic acid during the course of reaction meanwhile it is reduced to alcohol also.
The rate law states that rate of reaction is directly proportional to the Molar concentration of reactant each raised to some power. The power may or may not be equal to stoichiometric concentration of balanced chemical equations. The power to each reactant concentration is the individual order of reaction while the sum of all the power of concentration terms of reactant is overall order of reaction.
Also, for a complex reaction which occurs in more than one step the slowest step is the Rate determining step. Now from the reaction it is clear that the slow step of reaction is dependent on concentration of benzaldehyde and intermediate formed in the first step.
$Rate = {K_a}\left[ {PhCHO} \right]\left[ {Intermediate} \right] - - - - \left( 1 \right)$
The formation of intermediate is dependent on the concentration of on the equilibrium between Benzaldehyde and base.
${K_{eq}} = \dfrac{{\left[ {Intermediate} \right]}}{{\left[ {PhCHO} \right]\left[ {O{H^ - }} \right]}}$
Therefore, $\left[ {Intermediate} \right] = {K_{eq}}\left[ {PhCHO} \right]\left[ {O{H^ - }} \right] - - - - \left( 2 \right)$
Put value of equation 2 into equation 1:
$Rate = {K_a}\left[ {PhCHO} \right]{K_{eq}}\left[ {PhCHO} \right]\left[ {O{H^ - }} \right]$
$Rate = {K_a}{K_{eq}}{\left[ {PhCHO} \right]^2}\left[ {O{H^ - }} \right]$
Order of reaction = 2 + 1 = 3.
From the above equation it is clear that rate of Cannizzaro reaction depends on 2nd power of concentration of benzaldehyde and 1st power of concentration of Base. Hence overall Order of reaction is 3.
So, the correct answer is Option C.
Note: This question includes both slow step as well as equilibrium between intermediate and reactant. Also, here OH- is nucleophile and not base. Hence the rate of reaction depends on the nucleophile as well as reactants. Also, Involvement of two species in slow steps should not be misinterpreted as the reaction being second order as one part is intermediate and not any of the starting material hence should be replaced by any of the starting material.
Complete step by step solution:
The better insight into the mechanism of reaction can be obtained with study of kinetics. The Cannizzaro reaction is an example of a Disproportionation reaction in organic chemistry. In this reaction a non-enolizable aldehyde can be Oxidized as well as reduced simultaneously. An aldehyde is oxidised to carboxylic acid during the course of reaction meanwhile it is reduced to alcohol also.
The rate law states that rate of reaction is directly proportional to the Molar concentration of reactant each raised to some power. The power may or may not be equal to stoichiometric concentration of balanced chemical equations. The power to each reactant concentration is the individual order of reaction while the sum of all the power of concentration terms of reactant is overall order of reaction.
Also, for a complex reaction which occurs in more than one step the slowest step is the Rate determining step. Now from the reaction it is clear that the slow step of reaction is dependent on concentration of benzaldehyde and intermediate formed in the first step.
$Rate = {K_a}\left[ {PhCHO} \right]\left[ {Intermediate} \right] - - - - \left( 1 \right)$
The formation of intermediate is dependent on the concentration of on the equilibrium between Benzaldehyde and base.
${K_{eq}} = \dfrac{{\left[ {Intermediate} \right]}}{{\left[ {PhCHO} \right]\left[ {O{H^ - }} \right]}}$
Therefore, $\left[ {Intermediate} \right] = {K_{eq}}\left[ {PhCHO} \right]\left[ {O{H^ - }} \right] - - - - \left( 2 \right)$
Put value of equation 2 into equation 1:
$Rate = {K_a}\left[ {PhCHO} \right]{K_{eq}}\left[ {PhCHO} \right]\left[ {O{H^ - }} \right]$
$Rate = {K_a}{K_{eq}}{\left[ {PhCHO} \right]^2}\left[ {O{H^ - }} \right]$
Order of reaction = 2 + 1 = 3.
From the above equation it is clear that rate of Cannizzaro reaction depends on 2nd power of concentration of benzaldehyde and 1st power of concentration of Base. Hence overall Order of reaction is 3.
So, the correct answer is Option C.
Note: This question includes both slow step as well as equilibrium between intermediate and reactant. Also, here OH- is nucleophile and not base. Hence the rate of reaction depends on the nucleophile as well as reactants. Also, Involvement of two species in slow steps should not be misinterpreted as the reaction being second order as one part is intermediate and not any of the starting material hence should be replaced by any of the starting material.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




