
Explain what is meant by electrolysis of water. Write the electrode reactions and explain them.
Answer
573.6k+ views
Hint:Electrolysis of water is the decomposition into oxygen and hydrogen gas of water. We have to do electrolysis of water and write the equations for anode and cathode. Water $\left( {{H_2}O} \right)$ will decompose in ${O_2}$ and ${H_2}$ gases because water $\left( {{H_2}O} \right)$ use the composition of these two gases.
Complete answer:
Electrolysis is the process by which ionic substances are decomposed (broken down) into simpler substances when an electric current is passed through them.
Electricity is the flow of electrons or ions.
It is also called water splitting.
It requires a minimum potential difference of $1.23$ volts to split water.
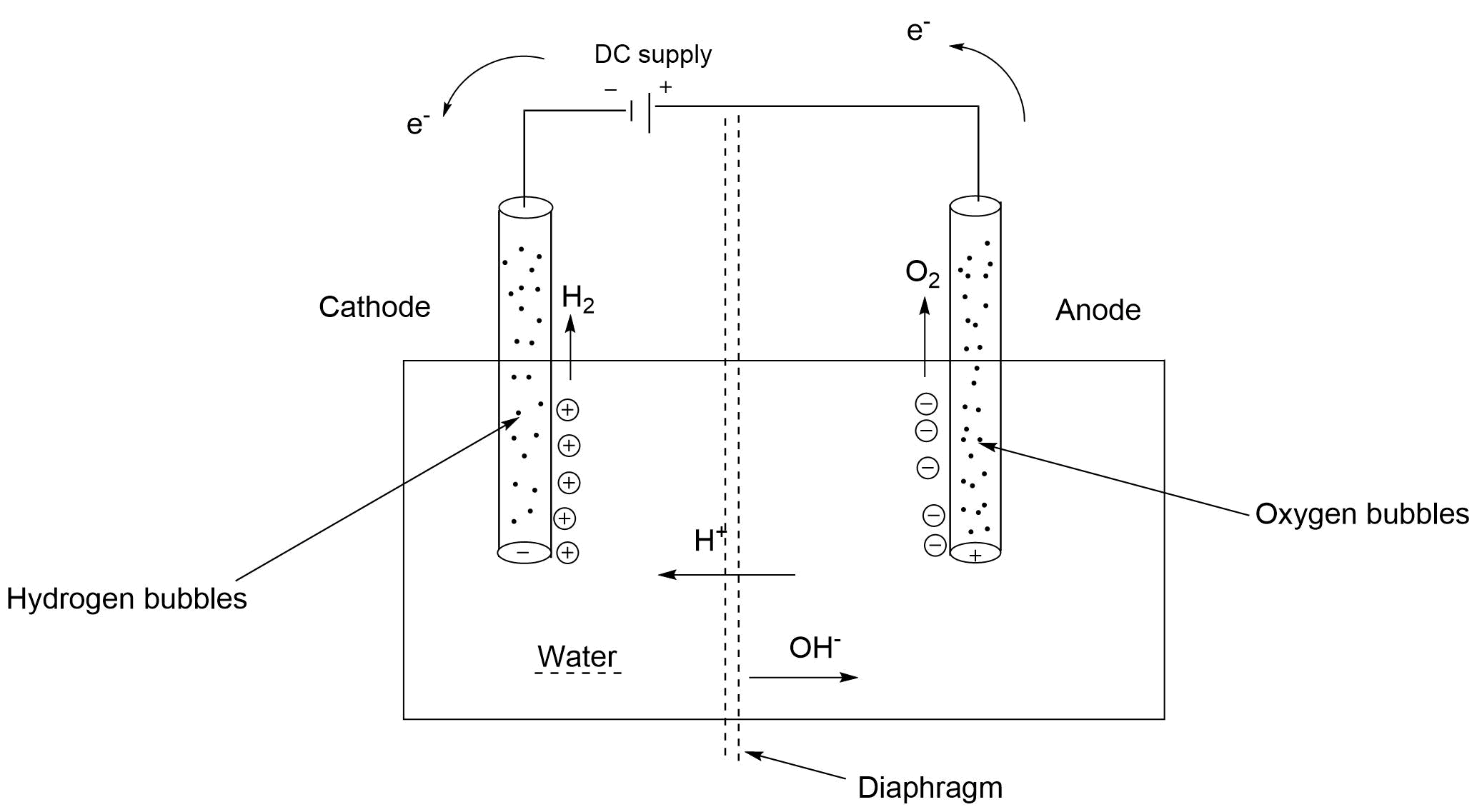
At anode:
\[2{H_2}{O_{(l)}}\xrightarrow{{}}{O_2}_{(g)} + 4{H^ + }_{(aq)} + 4{e^ - }\]
Or \[{{\text{H}}_2}{O_{(l)}}\xrightarrow{{}}2{H^ + } + \dfrac{1}{2}{O_2} + 2{e^ - }\]
At cathode:-
$2{H^ + } + 2{e^ - } \to {H_2}$
Net reaction:-${{\text{H}}_2}O \to {H_2} + \dfrac{1}{2}{O_2}$
When we apply $DC$ current in the $CKT$ then at anode \[{H^ + }\] ions will produce and they will attract cathode. \[{O_2}\] Gas will also be there.
At cathode: \[{H_2}\] gas will produce and \[O{H^ - }\] ions attract to anode.
We can see in the equation that the amount of \[{O_2}\] gas will help than \[{H_2}\] gas.
Volume of \[{H_2}\] is double than \[{O_2}_{(g)}\]
In the net reaction we get one mole of \[{H_2}_{(g)}\] and half mole of \[{O_2}_{(g)}\] from one mole of water.
The electrolyte and the electrodes used in electrolysis form an electrolyte, the positive ions of the electrolyte move toward the cathode (negative electrode), where they gain electrons to become a neutral substance.
Additional information: Uses of Electrolysis:
Electrolysis is commonly employed for coating one metal with another. The method of coating one metal with another using an electric current is called electroplating.
Note:
-After electrolysis of water, \[{H_2}_{(g)}\] is collected at negative cathode and \[{O_2}_{(g)}\] is collected at the positive anode.
-Hydrogen is double in volume than oxygen.
-Electrolysis water is one such most capable method for production of hydrogen because it uses renewable $\left( {{H_2}O} \right)$ and produces only pure oxygen as by product.
Complete answer:
Electrolysis is the process by which ionic substances are decomposed (broken down) into simpler substances when an electric current is passed through them.
Electricity is the flow of electrons or ions.
It is also called water splitting.
It requires a minimum potential difference of $1.23$ volts to split water.

At anode:
\[2{H_2}{O_{(l)}}\xrightarrow{{}}{O_2}_{(g)} + 4{H^ + }_{(aq)} + 4{e^ - }\]
Or \[{{\text{H}}_2}{O_{(l)}}\xrightarrow{{}}2{H^ + } + \dfrac{1}{2}{O_2} + 2{e^ - }\]
At cathode:-
$2{H^ + } + 2{e^ - } \to {H_2}$
Net reaction:-${{\text{H}}_2}O \to {H_2} + \dfrac{1}{2}{O_2}$
When we apply $DC$ current in the $CKT$ then at anode \[{H^ + }\] ions will produce and they will attract cathode. \[{O_2}\] Gas will also be there.
At cathode: \[{H_2}\] gas will produce and \[O{H^ - }\] ions attract to anode.
We can see in the equation that the amount of \[{O_2}\] gas will help than \[{H_2}\] gas.
Volume of \[{H_2}\] is double than \[{O_2}_{(g)}\]
In the net reaction we get one mole of \[{H_2}_{(g)}\] and half mole of \[{O_2}_{(g)}\] from one mole of water.
The electrolyte and the electrodes used in electrolysis form an electrolyte, the positive ions of the electrolyte move toward the cathode (negative electrode), where they gain electrons to become a neutral substance.
Additional information: Uses of Electrolysis:
Electrolysis is commonly employed for coating one metal with another. The method of coating one metal with another using an electric current is called electroplating.
Note:
-After electrolysis of water, \[{H_2}_{(g)}\] is collected at negative cathode and \[{O_2}_{(g)}\] is collected at the positive anode.
-Hydrogen is double in volume than oxygen.
-Electrolysis water is one such most capable method for production of hydrogen because it uses renewable $\left( {{H_2}O} \right)$ and produces only pure oxygen as by product.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




