
Explain the mechanism of the following reaction:
${\text{2C}}{{\text{H}}_3} - {\text{C}}{{\text{H}}_2} - {\text{OH}}\xrightarrow[{{\text{413 K}}}]{{{{\text{H}}^ + }}}{\text{C}}{{\text{H}}_3} - {\text{C}}{{\text{H}}_2} - {\text{O}} - {\text{C}}{{\text{H}}_2} - {\text{C}}{{\text{H}}_3} + {{\text{H}}_2}{\text{O}}$
Answer
579.9k+ views
Hint: In the reaction, the reactant has a hydroxyl $\left( { - {\text{OH}}} \right)$ functional group and the product has ether $\left( {{\text{R}} - {\text{O}} - {\text{R}}} \right)$ functional group, where R is any alkyl group. Thus, the reaction is conversion of alcohol to ether.
Complete step by step answer:
The given reaction is,
${\text{2C}}{{\text{H}}_3} - {\text{C}}{{\text{H}}_2} - {\text{OH}}\xrightarrow[{{\text{413 K}}}]{{{{\text{H}}^ + }}}{\text{C}}{{\text{H}}_3} - {\text{C}}{{\text{H}}_2} - {\text{O}} - {\text{C}}{{\text{H}}_2} - {\text{C}}{{\text{H}}_3} + {{\text{H}}_2}{\text{O}}$
In the given reaction, excess ethyl alcohol reacts with hydrogen ion from the sulphuric acid. In the reaction, diethyl ether is produced along with a water molecule.
The reaction mechanism involves three steps as follows:
Step 1: Formation of protonated alcohol:
In this step, one molecule of ethyl alcohol undergoes protonation i.e. a proton or hydrogen ion gets attached to the oxygen atom of the hydroxyl functional group. It is a fast step.
The reaction is as follows:
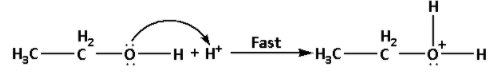
Step 2: Nucleophilic attack of the alcohol molecule on the protonated alcohol:
In this step, the second molecule of ethyl alcohol attacks the protonated alcohol molecule formed in the first step. It is a slow step.
The reaction is as follows:
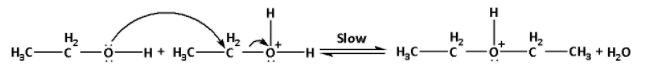
Step 3: Deprotonation to form ether:
In this step, the intermediate formed undergoes deprotonation i.e. a proton or a hydrogen ion gets removed. It is a fast step.
The reaction is as follows:
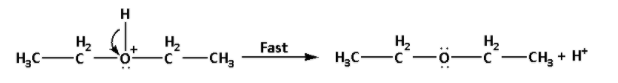
Note: In the reaction, equal volumes of ethyl alcohol and concentrated sulphuric acid are distilled. Ethyl alcohol reacts with concentrated sulphuric acid and produces ethyl hydrogen sulphate. The reaction is as follows:
${\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}} + {\text{H}} - {\text{O}} - {\text{S}}{{\text{O}}_{\text{3}}}{\text{H}} \to {\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{O}} - {\text{S}}{{\text{O}}_{\text{3}}}{\text{H}} + {{\text{H}}_2}{\text{O}}$
Then excess of ethyl alcohol is added. Ethyl hydrogen sulphate reacts with excess of ethyl alcohol and produces diethyl ether. The reaction is as follows:
\[{\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}} + {\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{O}} - {\text{S}}{{\text{O}}_{\text{3}}}{\text{H}} \to {\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{O}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{C}}{{\text{H}}_{\text{3}}} + {{\text{H}}_2}{\text{S}}{{\text{O}}_4}\]
The sulphuric acid is regenerated in the reaction. This process is known as a continuous esterification process.
Complete step by step answer:
The given reaction is,
${\text{2C}}{{\text{H}}_3} - {\text{C}}{{\text{H}}_2} - {\text{OH}}\xrightarrow[{{\text{413 K}}}]{{{{\text{H}}^ + }}}{\text{C}}{{\text{H}}_3} - {\text{C}}{{\text{H}}_2} - {\text{O}} - {\text{C}}{{\text{H}}_2} - {\text{C}}{{\text{H}}_3} + {{\text{H}}_2}{\text{O}}$
In the given reaction, excess ethyl alcohol reacts with hydrogen ion from the sulphuric acid. In the reaction, diethyl ether is produced along with a water molecule.
The reaction mechanism involves three steps as follows:
Step 1: Formation of protonated alcohol:
In this step, one molecule of ethyl alcohol undergoes protonation i.e. a proton or hydrogen ion gets attached to the oxygen atom of the hydroxyl functional group. It is a fast step.
The reaction is as follows:

Step 2: Nucleophilic attack of the alcohol molecule on the protonated alcohol:
In this step, the second molecule of ethyl alcohol attacks the protonated alcohol molecule formed in the first step. It is a slow step.
The reaction is as follows:

Step 3: Deprotonation to form ether:
In this step, the intermediate formed undergoes deprotonation i.e. a proton or a hydrogen ion gets removed. It is a fast step.
The reaction is as follows:

Note: In the reaction, equal volumes of ethyl alcohol and concentrated sulphuric acid are distilled. Ethyl alcohol reacts with concentrated sulphuric acid and produces ethyl hydrogen sulphate. The reaction is as follows:
${\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}} + {\text{H}} - {\text{O}} - {\text{S}}{{\text{O}}_{\text{3}}}{\text{H}} \to {\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{O}} - {\text{S}}{{\text{O}}_{\text{3}}}{\text{H}} + {{\text{H}}_2}{\text{O}}$
Then excess of ethyl alcohol is added. Ethyl hydrogen sulphate reacts with excess of ethyl alcohol and produces diethyl ether. The reaction is as follows:
\[{\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}} + {\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{O}} - {\text{S}}{{\text{O}}_{\text{3}}}{\text{H}} \to {\text{C}}{{\text{H}}_{\text{3}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{O}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{C}}{{\text{H}}_{\text{3}}} + {{\text{H}}_2}{\text{S}}{{\text{O}}_4}\]
The sulphuric acid is regenerated in the reaction. This process is known as a continuous esterification process.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




