
Explain the following reactions:
a) HVZ reaction
b) Trans-esterification reaction
c) Methyl Salicylate formation
Answer
577.2k+ views
Hint:. a) HVZ is a halogenation reaction.
b) Trans-esterification reaction results in, rather eponymously, the transformation of Carboxylic acid esters.
c) This reaction involves the methylation of salicylic acid to form Methyl Salicylate, also known as the Oil of Wintergreen.
Complete step by step answer:
Let us go through each of these reactions in detail one at a time so as to help facilitate better understanding of the subject.
a) While it is possible to brominate the alpha carbon of some carbonyl compounds such as aldehydes and ketones with $B{{r}_{2}}$ under certain acidic conditions, the reaction does not occur as a rule of thumb with other carbonyl compounds such as acids, esters, and amides because only aldehydes and ketones enolize to an acceptable enough extent to allow the reaction to occur. However, the alpha Carbon of these carbonyl compounds can be brominated with a mixture of $B{{r}_{2}}$ and $PB{{r}_{3}}$ in a reaction called the Hell-Volhard-Zelinsky (HVZ) reaction.
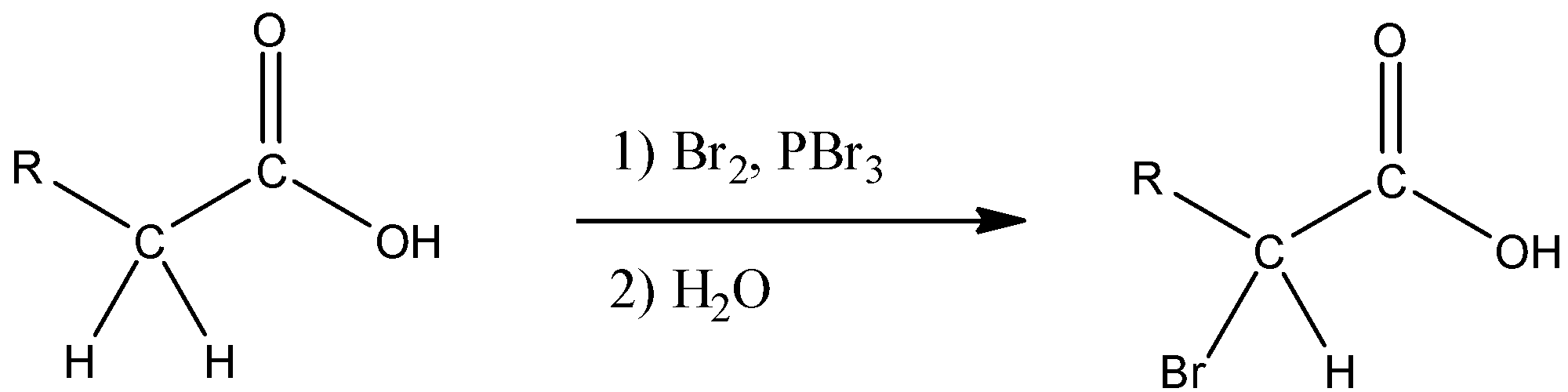
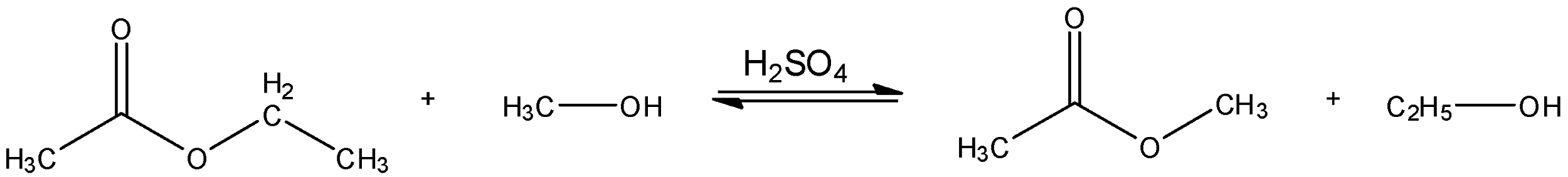
b) The process of conversion which turns the ester of a carboxylic acid into that of another carboxylic acid is referred to as transesterification. This reaction occurs when an ester is placed in the presence of an excess of alcohol along with that of an alkali or base and an ether which creates the possibility for an exchange in alkoxy groups. Per Le Chatelier’s principle, the greater the amount of base present, the faster the reaction moves forward.
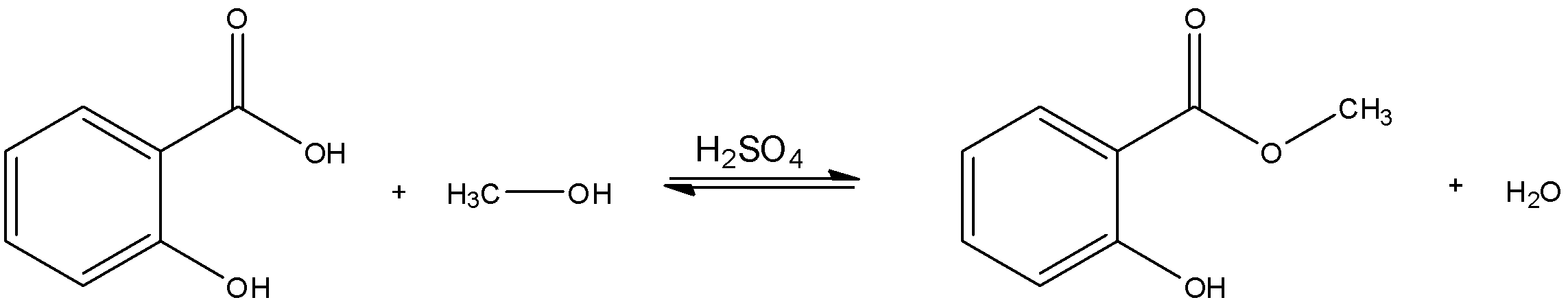
c) The synthesis of methyl salicylate ($C{{H}_{3}}COOC{{H}_{3}}$) is a type of condensation reaction as it involves the elimination of a water molecule from its reactants as the rest of its reactants (salicylic acid and$R-OH$) combine to give the final product with function group $-COOR$ . Since this is an esterification reaction at the end of the day, it requires the presence of excess acid for faster facilitation.
Note: Be very careful in the ordering of your answer as this is very important for a subjective question such as this with multiple parts. Also, remember that there are important catalysts involved for 2 of the 3 given reactions which facilitate faster reactions.
b) Trans-esterification reaction results in, rather eponymously, the transformation of Carboxylic acid esters.
c) This reaction involves the methylation of salicylic acid to form Methyl Salicylate, also known as the Oil of Wintergreen.
Complete step by step answer:
Let us go through each of these reactions in detail one at a time so as to help facilitate better understanding of the subject.
a) While it is possible to brominate the alpha carbon of some carbonyl compounds such as aldehydes and ketones with $B{{r}_{2}}$ under certain acidic conditions, the reaction does not occur as a rule of thumb with other carbonyl compounds such as acids, esters, and amides because only aldehydes and ketones enolize to an acceptable enough extent to allow the reaction to occur. However, the alpha Carbon of these carbonyl compounds can be brominated with a mixture of $B{{r}_{2}}$ and $PB{{r}_{3}}$ in a reaction called the Hell-Volhard-Zelinsky (HVZ) reaction.
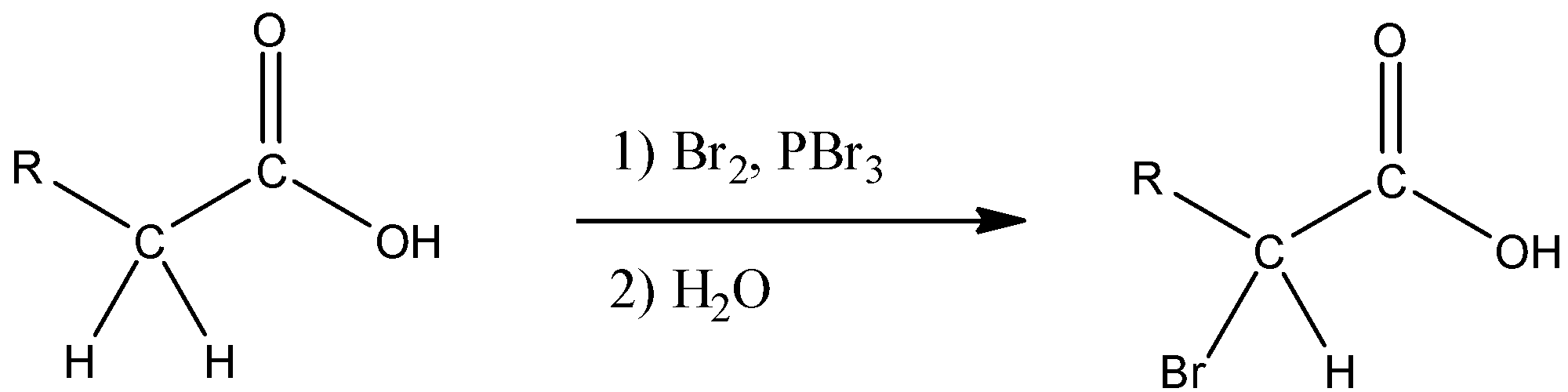
b) The process of conversion which turns the ester of a carboxylic acid into that of another carboxylic acid is referred to as transesterification. This reaction occurs when an ester is placed in the presence of an excess of alcohol along with that of an alkali or base and an ether which creates the possibility for an exchange in alkoxy groups. Per Le Chatelier’s principle, the greater the amount of base present, the faster the reaction moves forward.

c) The synthesis of methyl salicylate ($C{{H}_{3}}COOC{{H}_{3}}$) is a type of condensation reaction as it involves the elimination of a water molecule from its reactants as the rest of its reactants (salicylic acid and$R-OH$) combine to give the final product with function group $-COOR$ . Since this is an esterification reaction at the end of the day, it requires the presence of excess acid for faster facilitation.

Note: Be very careful in the ordering of your answer as this is very important for a subjective question such as this with multiple parts. Also, remember that there are important catalysts involved for 2 of the 3 given reactions which facilitate faster reactions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




