
Ethanol on reaction with acetic anhydride gives:
A. acetic ester
B. formic ester
C. ethanoic acid
D. ethyl acetate and ethanoic acid
Answer
597.3k+ views
Hint: It contains two molecules of an acid, with loss of a molecule of water. Addition of water to the anhydride regenerates two molecules of the carboxylic acid. The reaction between acetic anhydride and ethanol requires heating in the absence of catalysts.
Complete step by step answer:
The word anhydride means without water. Preparation of acetic anhydride is given in the reaction below:
\[{\text{R}} - {\text{COOH + R}} - {\text{COOH}} \rightleftarrows {\text{R}}{\left( {{\text{CO}}} \right)_2}{\text{O + }}{{\text{H}}_2}{\text{O}}\]
Carboxylic acid anhydride
Anhydrides composed of two different acids are called mixed anhydrides.
Acid anhydrides also known as acetic anhydrides, react with alcohol to produce esters and carboxylic acids. It does not require a catalyst, but still requires heating. The reaction is given below:
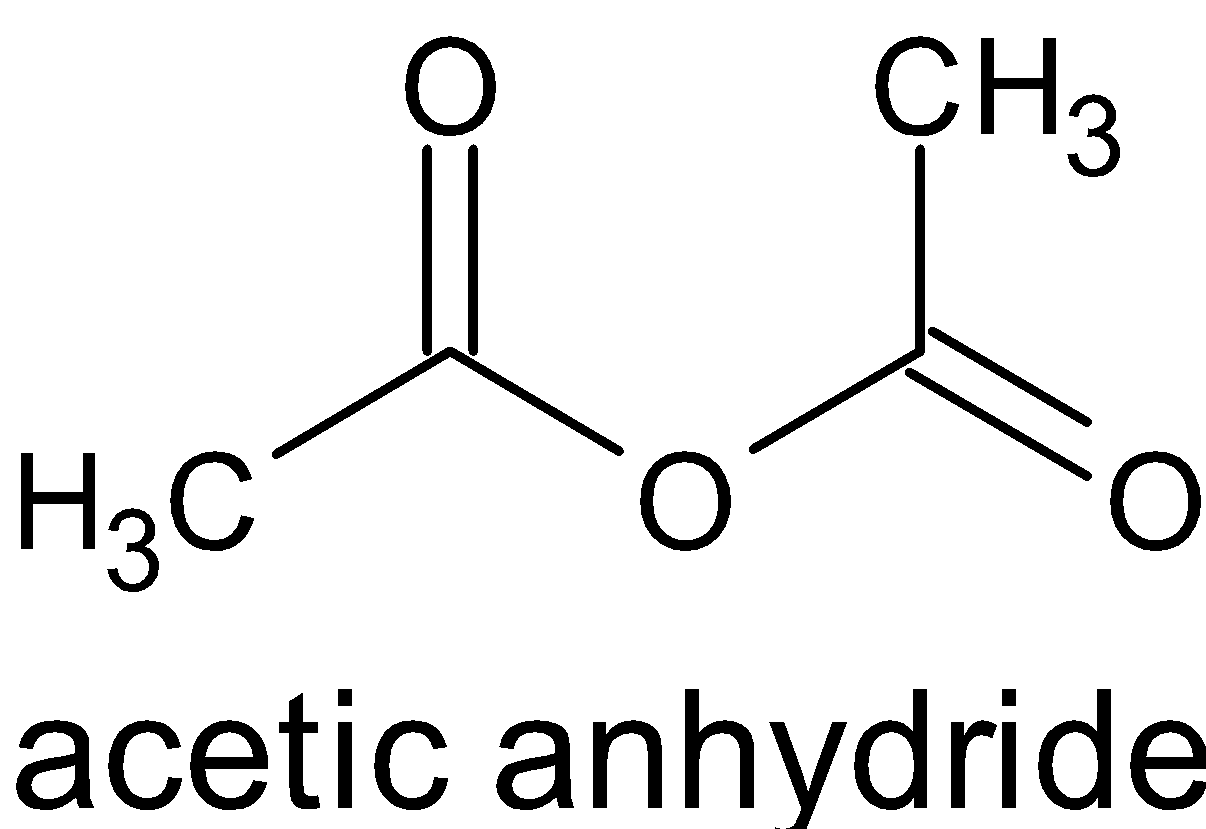 $ + {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}} \to $
$ + {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}} \to $
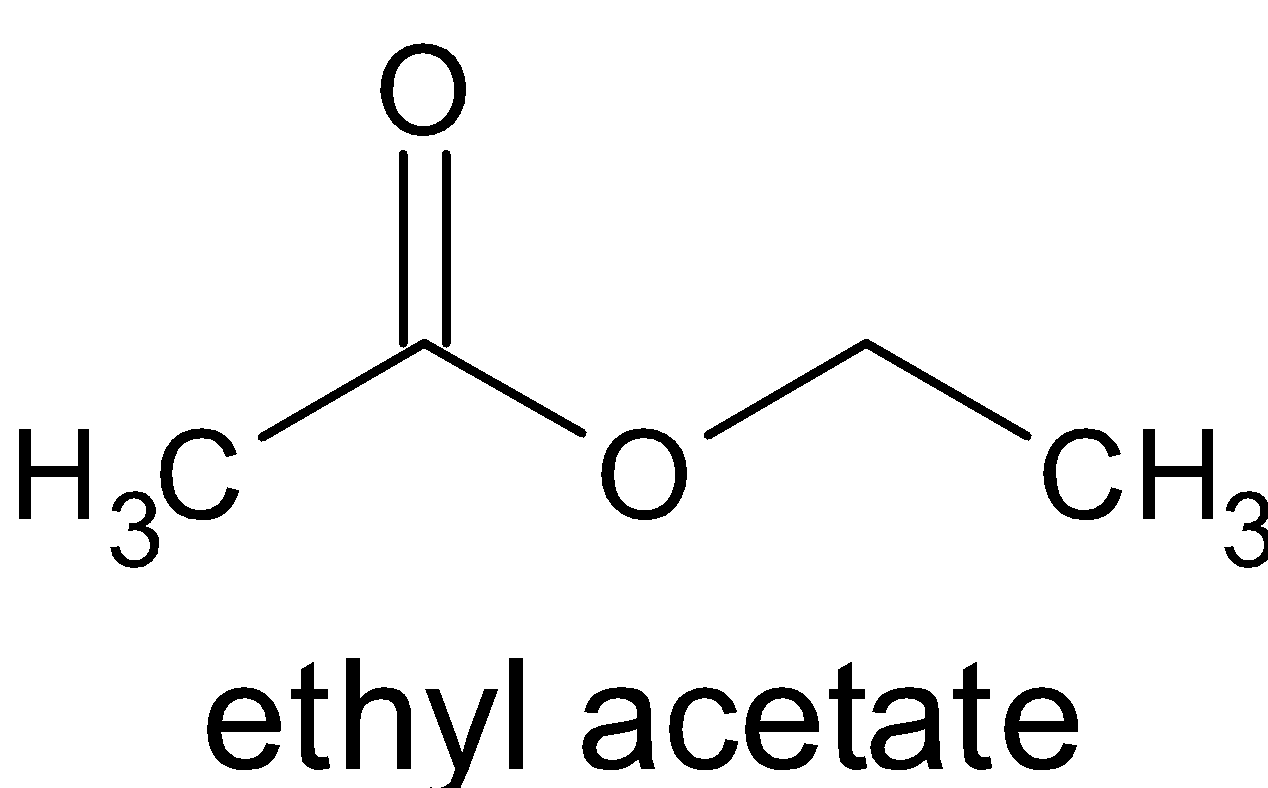 $ + {\text{C}}{{\text{H}}_3}{\text{COOH}}$
$ + {\text{C}}{{\text{H}}_3}{\text{COOH}}$
The mechanism involves four steps-nucleophilic attack by alcohol, deprotonation by pyridine, leaving group removal and protonation of carboxylate.
When acetic anhydride reacts with alcohol, it does not produce two molecules of ester, but an ester and ethanoic acid. While ethanoic acid reacts with alcohol, it produces ester. This does not occur with the acid which is formed by reacting with alcohol in the mixture. This is because this reaction occurs in the absence of catalysts. For the acids and alcohols to react, we need to heat and catalysts like sulfuric acid should be used.
So, the correct answer is option C.
Additional information Acetic anhydrides are not as reactive as acyl chlorides. When acyl chlorides react with alcohol, hydrochloric acid is produced instead of ethanoic acid.
Note:
In this reaction, pyridine acts as solvent. This reaction is similar when anhydrides react with water and phenol since all of them have hydroxyl groups. In water, the hydroxyl group is attached to hydrogen. In alcohols, hydroxyl groups are attached to alkyl groups. While in phenols, the hydroxyl group is attached to the benzene group.
Complete step by step answer:
The word anhydride means without water. Preparation of acetic anhydride is given in the reaction below:
\[{\text{R}} - {\text{COOH + R}} - {\text{COOH}} \rightleftarrows {\text{R}}{\left( {{\text{CO}}} \right)_2}{\text{O + }}{{\text{H}}_2}{\text{O}}\]
Carboxylic acid anhydride
Anhydrides composed of two different acids are called mixed anhydrides.
Acid anhydrides also known as acetic anhydrides, react with alcohol to produce esters and carboxylic acids. It does not require a catalyst, but still requires heating. The reaction is given below:


The mechanism involves four steps-nucleophilic attack by alcohol, deprotonation by pyridine, leaving group removal and protonation of carboxylate.
When acetic anhydride reacts with alcohol, it does not produce two molecules of ester, but an ester and ethanoic acid. While ethanoic acid reacts with alcohol, it produces ester. This does not occur with the acid which is formed by reacting with alcohol in the mixture. This is because this reaction occurs in the absence of catalysts. For the acids and alcohols to react, we need to heat and catalysts like sulfuric acid should be used.
So, the correct answer is option C.
Additional information Acetic anhydrides are not as reactive as acyl chlorides. When acyl chlorides react with alcohol, hydrochloric acid is produced instead of ethanoic acid.
Note:
In this reaction, pyridine acts as solvent. This reaction is similar when anhydrides react with water and phenol since all of them have hydroxyl groups. In water, the hydroxyl group is attached to hydrogen. In alcohols, hydroxyl groups are attached to alkyl groups. While in phenols, the hydroxyl group is attached to the benzene group.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




