
Energy required to break ethane into its constituent atoms is 2860 KJ. If the C-H bond energy is 420 KJ, the C-C bond energy is:-
Answer
592.8k+ views
Hint: The amount of energy released when one mole of bonds are formed from the isolated atoms or the energy required dissociating one mole of the bond. The energy required to break one mole is equal to the number of constituent bonds in the compound.
Complete answer:
Bond energy is the amount of energy released when one mole of bonds are formed from the isolated atoms in the gaseous state or the amount of energy required to dissociate one mole of bonds presents between the atoms in the gaseous molecules. It is represented by ${{\Delta }_{b}}H\text{ or }{{\Delta }_{bond}}H$ .
For diatomic molecules like ${{H}_{2}},{{O}_{2}},{{N}_{2}},C{{l}_{2}}$ etc, the bond energies are equal to their dissociation energies.
For polyatomic molecules, the bond energy of a particular bond is not the same. For example in the compound $C{{H}_{4}}$, there are 4 C-H bonds with different bond energies, i.e., +427, +439, +452, and +347 respectively. The enthalpy of atomization is equal to:
${{\Delta }_{a}}H(C{{H}_{4}})=427+439+52+347=1665KJ$
In such case average is taken:
${{\Delta }_{C-H}}=\dfrac{1665}{4}=416 KJ/mol$
So, the question ethane is dissociated into constituents as:
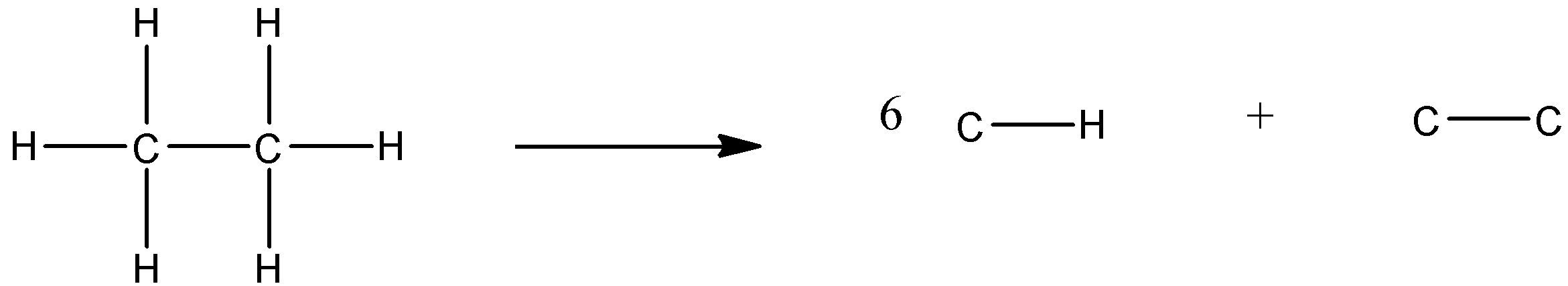
The enthalpy of atomization is given 2860 KJ and the bond energy of the C-H bond is 420KJ and the bond energy if C-C can be calculated by:
2860 = 6 (420) + C-C
C-C = 2860 – 2520
C-C = 340 KJ.
Hence the bond energy of the C-C bond is 340 KJ.
Note: The symbol used for bond dissociation enthalpy and mean bond enthalpy is the same. The number of the same bond must be multiplied in the equation. If different values of bond energy of the same bond are given, then the average value is considered.
Complete answer:
Bond energy is the amount of energy released when one mole of bonds are formed from the isolated atoms in the gaseous state or the amount of energy required to dissociate one mole of bonds presents between the atoms in the gaseous molecules. It is represented by ${{\Delta }_{b}}H\text{ or }{{\Delta }_{bond}}H$ .
For diatomic molecules like ${{H}_{2}},{{O}_{2}},{{N}_{2}},C{{l}_{2}}$ etc, the bond energies are equal to their dissociation energies.
For polyatomic molecules, the bond energy of a particular bond is not the same. For example in the compound $C{{H}_{4}}$, there are 4 C-H bonds with different bond energies, i.e., +427, +439, +452, and +347 respectively. The enthalpy of atomization is equal to:
${{\Delta }_{a}}H(C{{H}_{4}})=427+439+52+347=1665KJ$
In such case average is taken:
${{\Delta }_{C-H}}=\dfrac{1665}{4}=416 KJ/mol$
So, the question ethane is dissociated into constituents as:

The enthalpy of atomization is given 2860 KJ and the bond energy of the C-H bond is 420KJ and the bond energy if C-C can be calculated by:
2860 = 6 (420) + C-C
C-C = 2860 – 2520
C-C = 340 KJ.
Hence the bond energy of the C-C bond is 340 KJ.
Note: The symbol used for bond dissociation enthalpy and mean bond enthalpy is the same. The number of the same bond must be multiplied in the equation. If different values of bond energy of the same bond are given, then the average value is considered.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




