
During the formation of hydronium ions, water molecules act as acceptors. Enter 1 if the statement is True, else 0.
Answer
590.1k+ views
Hint: We have to know the molecule of water can be proton donor (or) a proton acceptor. If it is a proton donor, then the water molecule would act as Bronsted-Lowry acid. If a water molecule could accept a proton, then it acts as a Bronsted-Lowry base. Depending on the conditions, a molecule of water could play a role of acid and a base.
Complete step by step answer:
Now, let us define an acid.
A substance that donates protons (or) hydrogen ions is called an acid. Most acids are hydrogen atoms, which dissociates to form cation and anion in water.
Let us define a base.
A base is a substance, which neutralizes an acid. Base has the ability to accept a proton from an acid. Some of the bases such as metal hydroxides and metal oxide form neutral products on reaction with acids. Base is insoluble in water. Salts and water are produced when the base reacts with acids.
We have to know that hydronium ion is a significant factor when observing the chemical reactions, which takes place in aqueous solution. Its concentration related to hydroxide is a measure of pH of the solution. The formation of hydronium ion takes place only when there is acid present in water or just pure water. The chemical formula of hydronium ion is ${H_3}{O^ + }$. It could also be formed by the reaction of a hydrogen ion with molecules of water. We can write the chemical equation as,
In the formation of hydronium ions, water acts as a proton donor. So enter 0.
Note:
If we know the $pH$ of the solution, we can calculate the concentration of hydronium as,
${H_3}{O^ + } = {10^{ - pH}}$
If we have known concentration of hydronium, the $pH$ of the solution is calculated as,
$pH = - \log \left[ {{H_3}{O^ + }} \right]$
The geometry of hydronium ions is trigonal pyramidal and the electron geometry is tetrahedral. It contains three atoms of hydrogen and one atom of oxygen. The presence of a lone pair of oxygen gives the trigonal pyramidal shape to the molecule and bond angle between the atoms is ${113^ \circ }$.
We can draw the geometry of hydronium ion as,
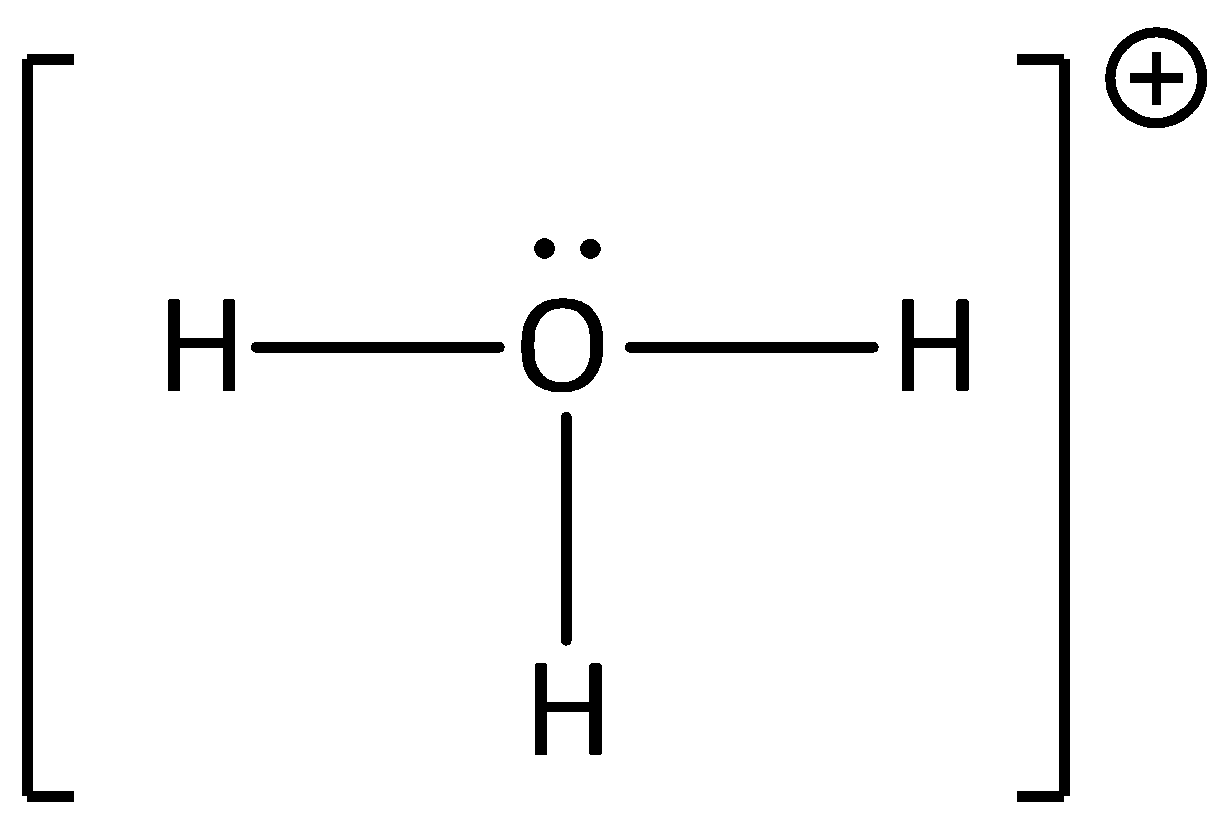
We have to know that hydrogen ions do not react in the aqueous solution, but they can be seen in the form of hydronium ions.
Complete step by step answer:
Now, let us define an acid.
A substance that donates protons (or) hydrogen ions is called an acid. Most acids are hydrogen atoms, which dissociates to form cation and anion in water.
Let us define a base.
A base is a substance, which neutralizes an acid. Base has the ability to accept a proton from an acid. Some of the bases such as metal hydroxides and metal oxide form neutral products on reaction with acids. Base is insoluble in water. Salts and water are produced when the base reacts with acids.
We have to know that hydronium ion is a significant factor when observing the chemical reactions, which takes place in aqueous solution. Its concentration related to hydroxide is a measure of pH of the solution. The formation of hydronium ion takes place only when there is acid present in water or just pure water. The chemical formula of hydronium ion is ${H_3}{O^ + }$. It could also be formed by the reaction of a hydrogen ion with molecules of water. We can write the chemical equation as,
In the formation of hydronium ions, water acts as a proton donor. So enter 0.
Note:
If we know the $pH$ of the solution, we can calculate the concentration of hydronium as,
${H_3}{O^ + } = {10^{ - pH}}$
If we have known concentration of hydronium, the $pH$ of the solution is calculated as,
$pH = - \log \left[ {{H_3}{O^ + }} \right]$
The geometry of hydronium ions is trigonal pyramidal and the electron geometry is tetrahedral. It contains three atoms of hydrogen and one atom of oxygen. The presence of a lone pair of oxygen gives the trigonal pyramidal shape to the molecule and bond angle between the atoms is ${113^ \circ }$.
We can draw the geometry of hydronium ion as,

We have to know that hydrogen ions do not react in the aqueous solution, but they can be seen in the form of hydronium ions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




