
Dry distillation of a mixture of calcium formate and calcium acetate gives: (more than one option is correct)
(A) HCHO
(B) $C{H_3}CHO$
(C) $C{H_3}COC{H_3}$
(D) None of these
Answer
597.3k+ views
Hint: Dry distillation causes a solid to change its phase from solid to liquid or gas. Calcium formate forms formaldehyde on dry distillation and calcium acetate forms acetone.
Complete answer:
-First let’s see what dry distillation is.
When solid materials are heated to produce gaseous products that can be condensed to solids or liquids, it is known as dry distillation. Pyrolysis and thermolysis are involved in this process.
When dry distillation causes chemical changes to occur it is known as destructive distillation or cracking. If there is only phase change and no chemical change it is classical distillation.
-Calcium formate: It is calcium salt of formic acid. It’s molecular formula is: $Ca{(HCOO)_2}$. It undergoes dry distillation to form formaldehyde ($HCHO$).
 -Calcium acetate: It is a calcium salt of acetic acid. It’s molecular formula is: ${(C{H_3}COO)_2}Ca$. It undergoes dry distillation to form acetone ($C{H_3}COC{H_3}$ ).
-Calcium acetate: It is a calcium salt of acetic acid. It’s molecular formula is: ${(C{H_3}COO)_2}Ca$. It undergoes dry distillation to form acetone ($C{H_3}COC{H_3}$ ).
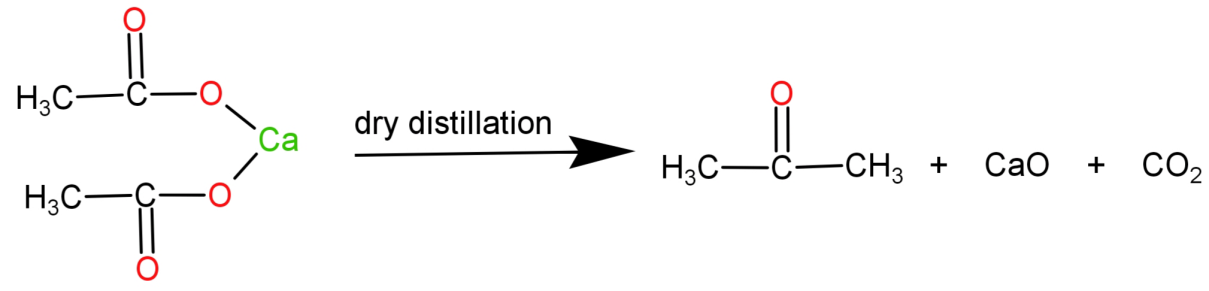 -When both calcium formate and calcium acetate undergo dry distillation together they produce a mixture of products. They produce acetaldehyde, acetone and formaldehyde.
-When both calcium formate and calcium acetate undergo dry distillation together they produce a mixture of products. They produce acetaldehyde, acetone and formaldehyde.
The reaction can be written as:

Therefore acetaldehyde, formaldehyde and acetone are formed by the dry distillation of this mixture.
So, the correct options are:
(A) HCHO
(B) $C{H_3}CHO$
(C) $C{H_3}COC{H_3}$
Note: Sometimes we think that only the 2 products which are formed during the separate dry distillations of calcium acetate and calcium formate will be formed during the dry distillation of the mixture. So, remember that these 2 salts together produce a mixed product acetaldehyde also.
Complete answer:
-First let’s see what dry distillation is.
When solid materials are heated to produce gaseous products that can be condensed to solids or liquids, it is known as dry distillation. Pyrolysis and thermolysis are involved in this process.
When dry distillation causes chemical changes to occur it is known as destructive distillation or cracking. If there is only phase change and no chemical change it is classical distillation.
-Calcium formate: It is calcium salt of formic acid. It’s molecular formula is: $Ca{(HCOO)_2}$. It undergoes dry distillation to form formaldehyde ($HCHO$).


The reaction can be written as:

Therefore acetaldehyde, formaldehyde and acetone are formed by the dry distillation of this mixture.
So, the correct options are:
(A) HCHO
(B) $C{H_3}CHO$
(C) $C{H_3}COC{H_3}$
Note: Sometimes we think that only the 2 products which are formed during the separate dry distillations of calcium acetate and calcium formate will be formed during the dry distillation of the mixture. So, remember that these 2 salts together produce a mixed product acetaldehyde also.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




