
Draw the structures of (a) $Mn{{O}_{4}}$ (b) $C{{r}_{2}}{{O}_{7}}^{2-}$
Answer
554.1k+ views
Hint: Manganate and chromate ion follow the VSEPR theory when we draw its structure. Both the ions given in the question can act as oxidising agents as they have extra electrons, and we know that oxidising agents are those which itself gets reduced.
Complete step by step answer:
The structures of the given compounds could be drawn by the use of VSEPR theory. The full form of VSEPR theory is valence shell electron pair repulsion theory. The postulates may include: In polyatomic molecules, which means the molecules having more than one atom, one of the constituent atoms is recognized as the central atom to which all other atoms which are a part of the molecule are attached. The total number of valence shell electron pairs decides the shape of the molecule.
We know that the electron pairs have a tendency to orient themselves in a way that minimizes the repulsion between the electrons and maximizes the distance between them.
We can consider the valence shell as a sphere in which the electron pairs are localized on the surface in such a way that the distance between them is maximized.
If we surround the central atom of the molecule by bond pairs of electrons, then, the shape of the molecule would be asymmetrical.
And if the central atom is surrounded by both the bond pairs and lone pairs of electrons, then the molecule would have a distorted shape.
We can apply VSEPR theory to each structure of resonance of a molecule.
The repulsive strength is strongest in two lone pairs and weakest in two bond pairs.
If electron pairs which are present around the central atom are closer to one other, they tend to repel each other. This results in an increase in the energy of the molecules.
If the pairs of electrons lie far from each other, the repulsions which will be experienced by both of them will be less and ultimately, the overall energy of the molecule will be low.
The structure of $Mn{{O}_{4}}$ is given below.
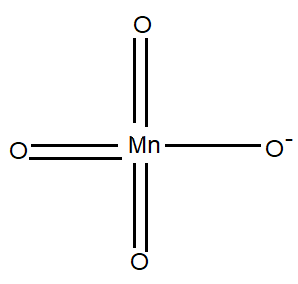
The structure of $C{{r}_{2}}{{O}_{7}}^{2-}$ is given below.
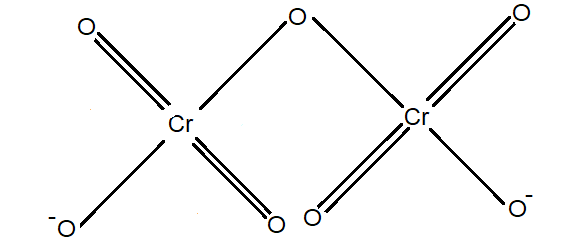
Note: Manganates, specifically the insoluble barium manganate, have been used as oxidizing agents in synthesis of organic compounds: they tend to oxidize primary alcohols to aldehydes and then they convert it into carboxylic acids, and secondary alcohols to ketones.
Dichromate on the other hand is widely used in the laboratory as reagents to perform various chemical reactions. It has a significant role in titration, as they are fairly strong oxidising agents.
Complete step by step answer:
The structures of the given compounds could be drawn by the use of VSEPR theory. The full form of VSEPR theory is valence shell electron pair repulsion theory. The postulates may include: In polyatomic molecules, which means the molecules having more than one atom, one of the constituent atoms is recognized as the central atom to which all other atoms which are a part of the molecule are attached. The total number of valence shell electron pairs decides the shape of the molecule.
We know that the electron pairs have a tendency to orient themselves in a way that minimizes the repulsion between the electrons and maximizes the distance between them.
We can consider the valence shell as a sphere in which the electron pairs are localized on the surface in such a way that the distance between them is maximized.
If we surround the central atom of the molecule by bond pairs of electrons, then, the shape of the molecule would be asymmetrical.
And if the central atom is surrounded by both the bond pairs and lone pairs of electrons, then the molecule would have a distorted shape.
We can apply VSEPR theory to each structure of resonance of a molecule.
The repulsive strength is strongest in two lone pairs and weakest in two bond pairs.
If electron pairs which are present around the central atom are closer to one other, they tend to repel each other. This results in an increase in the energy of the molecules.
If the pairs of electrons lie far from each other, the repulsions which will be experienced by both of them will be less and ultimately, the overall energy of the molecule will be low.
The structure of $Mn{{O}_{4}}$ is given below.

The structure of $C{{r}_{2}}{{O}_{7}}^{2-}$ is given below.

Note: Manganates, specifically the insoluble barium manganate, have been used as oxidizing agents in synthesis of organic compounds: they tend to oxidize primary alcohols to aldehydes and then they convert it into carboxylic acids, and secondary alcohols to ketones.
Dichromate on the other hand is widely used in the laboratory as reagents to perform various chemical reactions. It has a significant role in titration, as they are fairly strong oxidising agents.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




