
Draw the structure of xenon hexafluoride ($Xe{{F}_{6}}$) molecule and state the hybridization of the central atom.
Answer
600k+ views
Hint: The oxidation state of Xe in $Xe{{F}_{6}}$ is +6. $Xe{{F}_{6}}$ is a crystalline solid with melting point 49.5$^{o}C$. It is the most volatile of all the fluorides of Xe and its vapour has a greenish yellow colour.
Complete answer:
Electronic combination of Xe (54) in the ground state is given as:
Xe (in ground state) : $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}4{{s}^{2}}4{{p}^{6}}4{{d}^{10}}5{{s}^{2}}5{{p}^{6}}$
Outer shell configuration of Xe is $5{{s}^{2}}5{{p}^{6}}$.
$Xe{{F}_{6}}$ has six fluoride atoms. So, the total number of electrons on Xe is (8+6) =14.
We know that one pair of electrons contains 2 electrons. Therefore, the total number of electrons pairs available is 7.
There are six bonds present in$Xe{{F}_{6}}$. So, 3 electrons from the 5p – orbitals move into 5d orbitals so that six electrons can be made available for bonding. Seven orbitals (one 5s+three 5p+three 5d) hybridize to give $s{{p}^{3}}{{d}^{3}}$ hybridization.
Now, outer shell electronic combination of Xe in +6 oxidation state has become $5{{s}^{2}}5{{p}^{3}}5{{d}^{3}}$.
Six out of the seven orbitals of Xe are used in bonding with six fluorine atoms. One orbital accommodates a pair of electrons.
Valence shell electrons pair theory (VESPER) suggests that the shape of the molecule depends on the total number of electrons including bonding and lone pairs in the valence shell of the Xe atom.
The hybridization of Xe in $Xe{{F}_{6}}$ is $s{{p}^{3}}{{d}^{3}}$. Due to $s{{p}^{3}}{{d}^{3}}$ hybridization, $Xe{{F}_{6}}$ should have pentagonal bipyramidal geometry as shown below. However, no evidence has been provided to prove the existence of this structure.
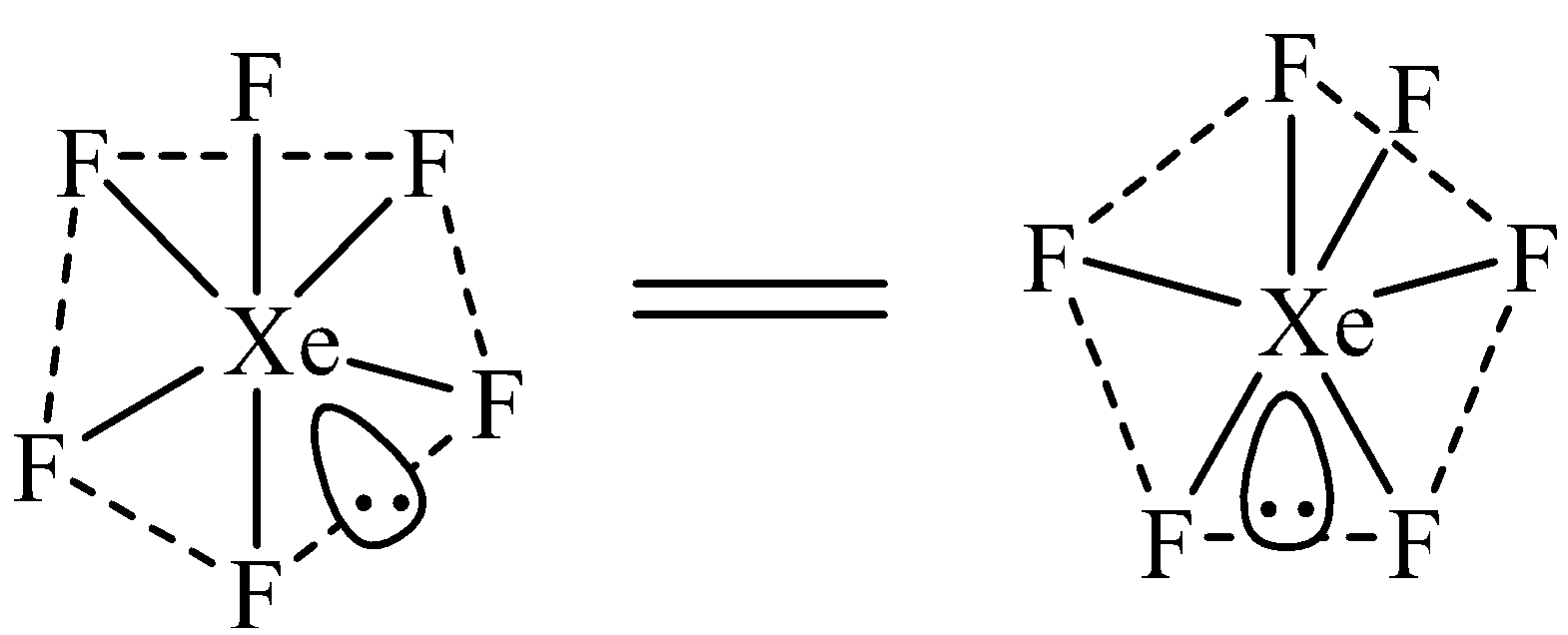
$Xe{{F}_{6}}$ has been suggested to have a distorted octahedral structure in which the six positions are occupied by fluorine atoms forming an octahedral and one lone pair is present at the center of one of the triangular faces. Distorted octahedral structure of \[Xe{{F}_{6}}\] is given below.
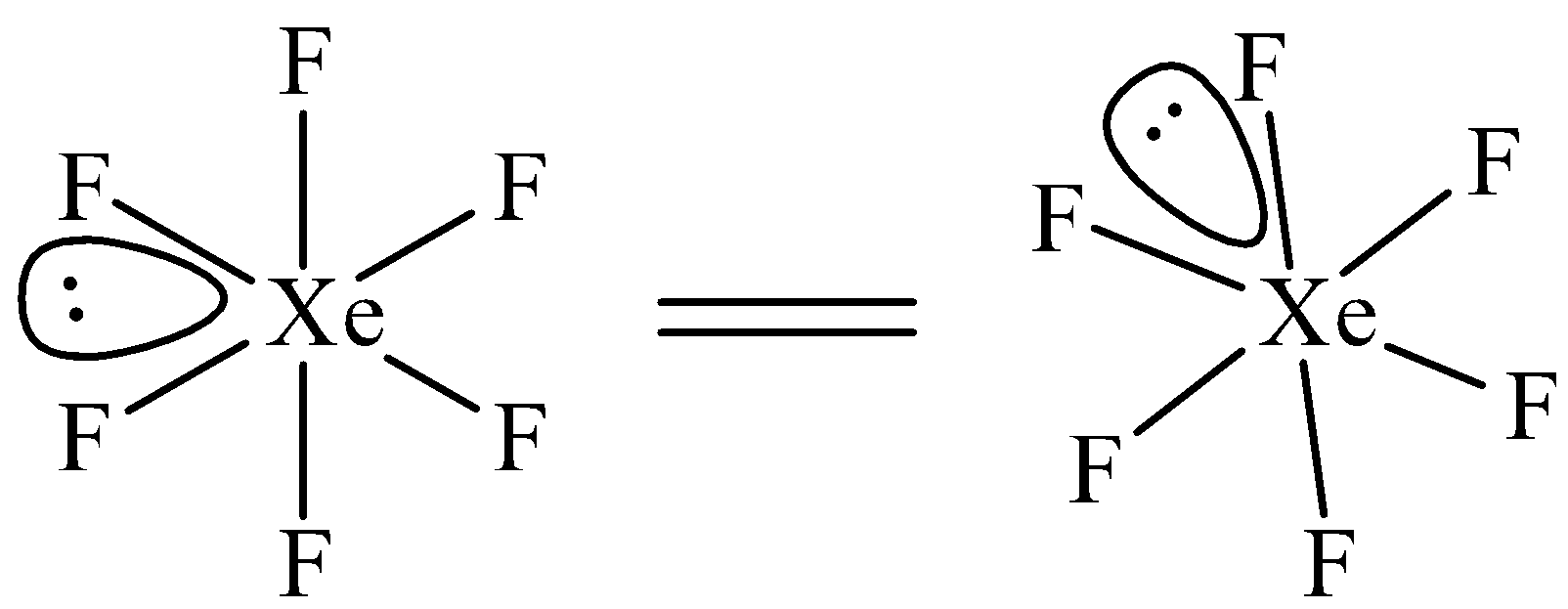
Note: Structure of $Xe{{F}_{6}}$ is controversial. Remember the structure of $Xe{{F}_{6}}$ as an exception. In $Xe{{F}_{6}}$, number of bond pairs (Xe-F) = 6 and number of lone pairs =1.
Complete answer:
Electronic combination of Xe (54) in the ground state is given as:
Xe (in ground state) : $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}4{{s}^{2}}4{{p}^{6}}4{{d}^{10}}5{{s}^{2}}5{{p}^{6}}$
Outer shell configuration of Xe is $5{{s}^{2}}5{{p}^{6}}$.
$Xe{{F}_{6}}$ has six fluoride atoms. So, the total number of electrons on Xe is (8+6) =14.
We know that one pair of electrons contains 2 electrons. Therefore, the total number of electrons pairs available is 7.
There are six bonds present in$Xe{{F}_{6}}$. So, 3 electrons from the 5p – orbitals move into 5d orbitals so that six electrons can be made available for bonding. Seven orbitals (one 5s+three 5p+three 5d) hybridize to give $s{{p}^{3}}{{d}^{3}}$ hybridization.
Now, outer shell electronic combination of Xe in +6 oxidation state has become $5{{s}^{2}}5{{p}^{3}}5{{d}^{3}}$.
Six out of the seven orbitals of Xe are used in bonding with six fluorine atoms. One orbital accommodates a pair of electrons.
Valence shell electrons pair theory (VESPER) suggests that the shape of the molecule depends on the total number of electrons including bonding and lone pairs in the valence shell of the Xe atom.
The hybridization of Xe in $Xe{{F}_{6}}$ is $s{{p}^{3}}{{d}^{3}}$. Due to $s{{p}^{3}}{{d}^{3}}$ hybridization, $Xe{{F}_{6}}$ should have pentagonal bipyramidal geometry as shown below. However, no evidence has been provided to prove the existence of this structure.
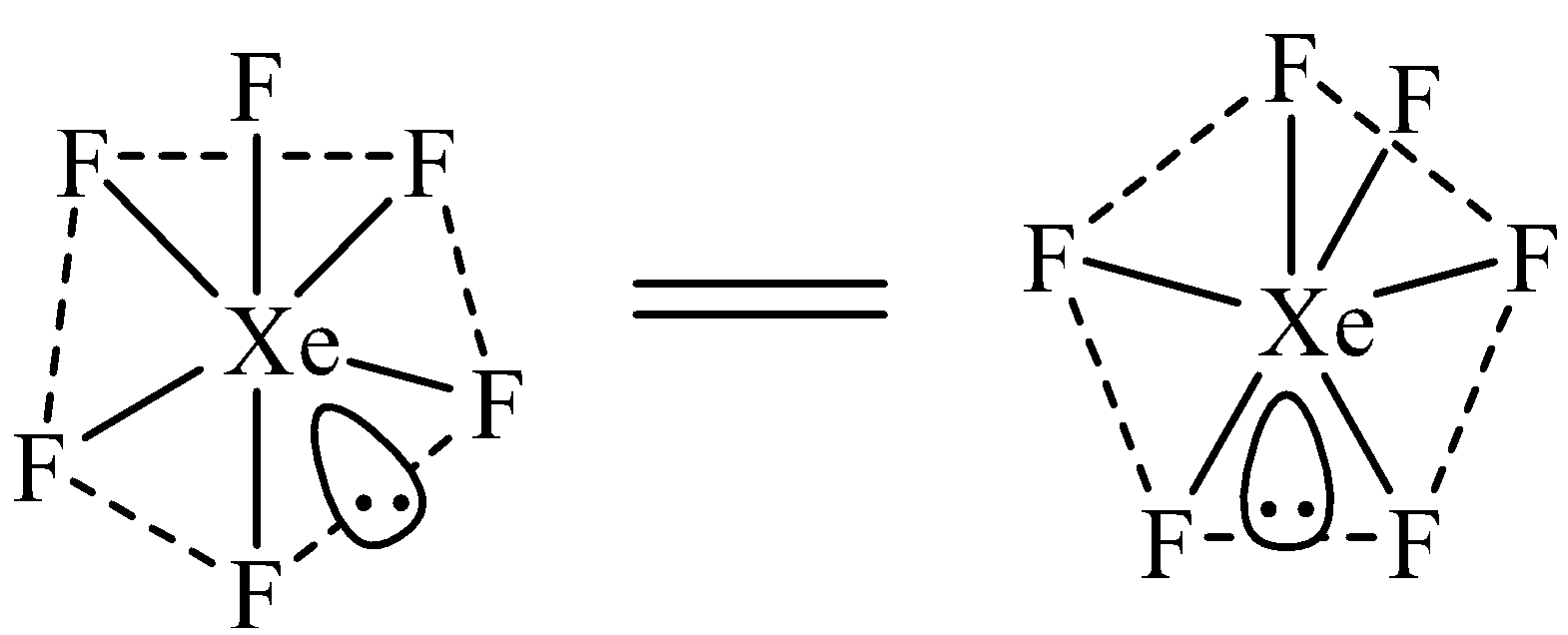
$Xe{{F}_{6}}$ has been suggested to have a distorted octahedral structure in which the six positions are occupied by fluorine atoms forming an octahedral and one lone pair is present at the center of one of the triangular faces. Distorted octahedral structure of \[Xe{{F}_{6}}\] is given below.
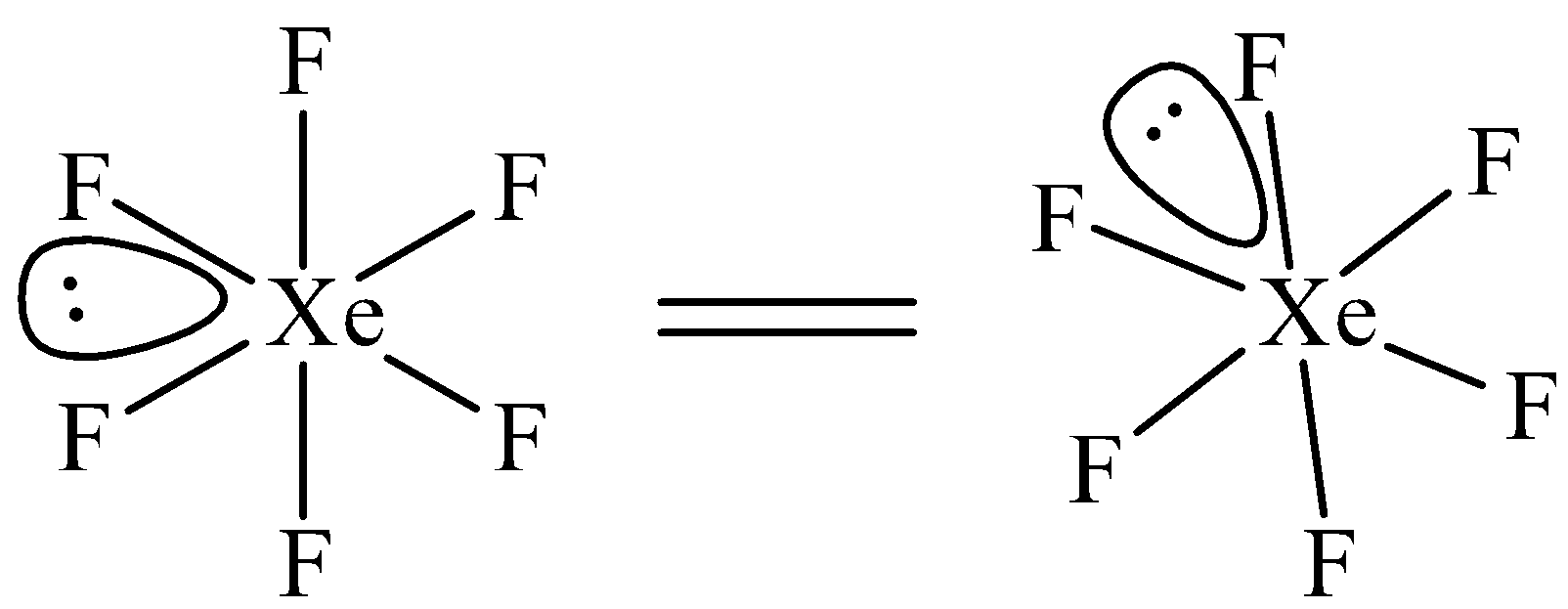
Note: Structure of $Xe{{F}_{6}}$ is controversial. Remember the structure of $Xe{{F}_{6}}$ as an exception. In $Xe{{F}_{6}}$, number of bond pairs (Xe-F) = 6 and number of lone pairs =1.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




