
Draw the structure of the following:
$2-methylbutane$, $prop-1-yne$, $pent-2-ene$.
Answer
573.6k+ views
Hint: In order to draw the structure of a compound from a given name, we should first see the root word which tells us about the number of carbon in the skeletal structure.
In case of $2-methylbutane$, its butane which means the skeletal structure should have four carbons, similarly in case of $prop-1-yne$ and $pent-2-ene$, its prop and pent which means that the skeletal structures would have three and five carbons respectively.
Complete step by step answer:
If we consider the name of the compound given in the question, we can see that it is written in IUPAC nomenclature.
IUPAC stands for International Union of Pure and Applied Chemistry. IUPAC naming of organic compounds is a systematic naming, by using certain specific rules. These rules are listed below.
First we identify the longest carbon chain. This longest carbon chain is termed as the parent chain.
Next we identify all of the substituents, which are attached to that carbon chain through bonds. We call it functional groups.
Now we number the carbons of the parent chain that is the longest chain, from the end which gives the lowest numbers to the substituents. If two or more side chains are present at equivalent positions, assign the lowest number according to the alphabetical order, as in the substituent whose name comes up first alphabetically, will be numbered first.
If the same substituent comes up more than once, we are supposed to write the location of each point on which the substituent is attached. Also, we are supposed to write the number of times the substituent group is coming up, by a prefix (di, tri, tetra, etc.).
If there are two or more different substituents they are listed in alphabetical order using the name of the base. The prefixes such as sec- and tert- are not used in determining the alphabetical order except for the case when they are compared with each other.
The structure of $2-methylbu\tan e$, $prop-1-yne$, $pent-2-ene$ are given below.
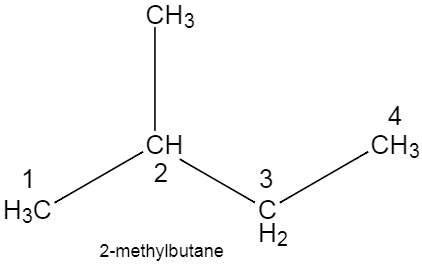
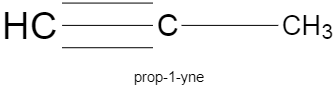
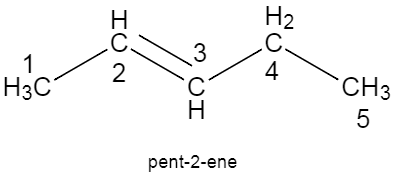
Note: The most common mistake while writing the structure of a compound from the given name, is when we get confused regarding the numbering of the carbon atom. To avoid this, you could always write the structure by roughly writing the numbers of carbon atoms at the beginning of the process.Note how the lowest number is given to the double bond in the structure of pentene, and triple bond in case of propyne.
In case of $2-methylbutane$, its butane which means the skeletal structure should have four carbons, similarly in case of $prop-1-yne$ and $pent-2-ene$, its prop and pent which means that the skeletal structures would have three and five carbons respectively.
Complete step by step answer:
If we consider the name of the compound given in the question, we can see that it is written in IUPAC nomenclature.
IUPAC stands for International Union of Pure and Applied Chemistry. IUPAC naming of organic compounds is a systematic naming, by using certain specific rules. These rules are listed below.
First we identify the longest carbon chain. This longest carbon chain is termed as the parent chain.
Next we identify all of the substituents, which are attached to that carbon chain through bonds. We call it functional groups.
Now we number the carbons of the parent chain that is the longest chain, from the end which gives the lowest numbers to the substituents. If two or more side chains are present at equivalent positions, assign the lowest number according to the alphabetical order, as in the substituent whose name comes up first alphabetically, will be numbered first.
If the same substituent comes up more than once, we are supposed to write the location of each point on which the substituent is attached. Also, we are supposed to write the number of times the substituent group is coming up, by a prefix (di, tri, tetra, etc.).
If there are two or more different substituents they are listed in alphabetical order using the name of the base. The prefixes such as sec- and tert- are not used in determining the alphabetical order except for the case when they are compared with each other.
The structure of $2-methylbu\tan e$, $prop-1-yne$, $pent-2-ene$ are given below.



Note: The most common mistake while writing the structure of a compound from the given name, is when we get confused regarding the numbering of the carbon atom. To avoid this, you could always write the structure by roughly writing the numbers of carbon atoms at the beginning of the process.Note how the lowest number is given to the double bond in the structure of pentene, and triple bond in case of propyne.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




