
Draw the structure of benzaldehyde $-2,4-$ dinitrophenylhydrazone
Answer
573.9k+ views
Hint: The name phenylhydrazone is derived from phenylhydrazine, which contains two amine groups attached to each other with a single bond and one of the groups has an attachment with a carbon atom of benzene ring, hence the root name phenyl. The valency of the other amine group is satisfied by two hydrogen atoms.
Complete step by step answer:
If we consider the name of the compound given in the question, we can see that it is written in IUPAC nomenclature.
IUPAC stands for International Union of Pure and Applied Chemistry. IUPAC naming of organic compounds is a systematic naming, by using certain specific rules. These rules are listed below.
First we identify the longest carbon chain. This longest carbon chain is termed as the parent chain.
Next we identify all of the substituents, which are attached to that carbon chain through bonds. We call it functional groups.
Now we number the carbons of the parent chain that is the longest chain, from the end which gives the lowest numbers to the substituents. If two or more side chains are present at equivalent positions, assign the lowest number according to the alphabetical order, as in the substituent whose name comes up first alphabetically, will be numbered first.
If the same substituent comes up more than once, we are supposed to write the location of each point on which the substituent is attached. Also, we are supposed to write the number of times the substituent group is coming up, by a prefix (di, tri, tetra, etc.).
If there are two or more different substituents they are listed in alphabetical order using the name of the base. The prefixes such as sec- and tert- are not used in determining the alphabetical order except for the case when they are compared with each other.
Now, the compound given to us has two benzene rings, but we will prioritize the one having more number of substituents.
The structure of benzaldehyde $-2,4-$ dinitrophenylhydrazone is given below.
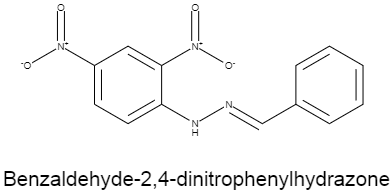
As we can see the numbering of the benzene group which is having two nitro groups attached to it, starts from the carbon, with which the phenylhydrazone group is attached. As it is a bigger group, it gets to have the lower number according to the IUPAC naming system of organic compounds.
Note: The benzaldehyde $-2,4-$ dinitrophenylhydrazone is a substituted hydrazine compound generally used to test the presence of aldehydes and ketones in a given organic compound which is unknown to us.
Complete step by step answer:
If we consider the name of the compound given in the question, we can see that it is written in IUPAC nomenclature.
IUPAC stands for International Union of Pure and Applied Chemistry. IUPAC naming of organic compounds is a systematic naming, by using certain specific rules. These rules are listed below.
First we identify the longest carbon chain. This longest carbon chain is termed as the parent chain.
Next we identify all of the substituents, which are attached to that carbon chain through bonds. We call it functional groups.
Now we number the carbons of the parent chain that is the longest chain, from the end which gives the lowest numbers to the substituents. If two or more side chains are present at equivalent positions, assign the lowest number according to the alphabetical order, as in the substituent whose name comes up first alphabetically, will be numbered first.
If the same substituent comes up more than once, we are supposed to write the location of each point on which the substituent is attached. Also, we are supposed to write the number of times the substituent group is coming up, by a prefix (di, tri, tetra, etc.).
If there are two or more different substituents they are listed in alphabetical order using the name of the base. The prefixes such as sec- and tert- are not used in determining the alphabetical order except for the case when they are compared with each other.
Now, the compound given to us has two benzene rings, but we will prioritize the one having more number of substituents.
The structure of benzaldehyde $-2,4-$ dinitrophenylhydrazone is given below.

As we can see the numbering of the benzene group which is having two nitro groups attached to it, starts from the carbon, with which the phenylhydrazone group is attached. As it is a bigger group, it gets to have the lower number according to the IUPAC naming system of organic compounds.
Note: The benzaldehyde $-2,4-$ dinitrophenylhydrazone is a substituted hydrazine compound generally used to test the presence of aldehydes and ketones in a given organic compound which is unknown to us.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




