
Draw the structure of:
A.Pyrophosphoric acid
B.Pyro phosphorous acid
C.Poly metaphosphoric acid
D.Cyclo trimetaphosphoric acid
Answer
582.9k+ views
Hint: To answer this question, you should recall the concept of oxoacids of phosphorus. Phosphorus belongs to group 15 of the periodic table and forms various oxoacids: hypo phosphorous acid\[\left( {{H_3}P{O_2}} \right)\], Phosphorous acid\[\left( {{H_3}P{O_3}} \right)\], Hypo phosphoric acid\[\left( {{H_3}P{O_4}} \right)\], pyro phosphoric acid \[\left( {{H_4}{P_2}{O_7}} \right)\]and poly metaphosphoric acid\[{\left( {HP{O_3}} \right)_n}\].
Complete Step by step solution:
Oxoacids of phosphorus which contain phosphorus-oxygen linkages are the most dominated subset in Phosphorus Chemistry.
Pyro phosphoric acid is a tetrabasic acid and prepared by heating orthophosphoric acid at about \[{250^o}{\text{C}}\] : \[2{H_3}P{O_4} \to {H_4}{P_2}{O_7} + {H_2}O\] The structure of Pyro phosphoric acid can be represented as:
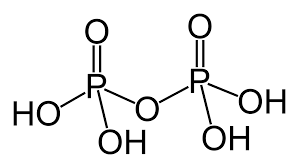
The chemical formula of pyrophosphoric acid is \[{H_4}{P_2}{O_5}\]. The structure can be represented as:
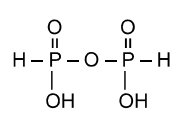
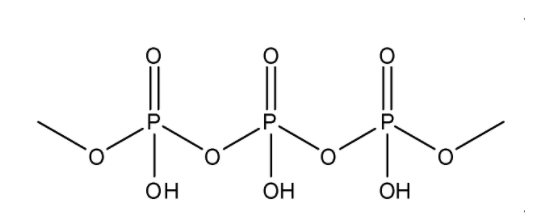
The formula for Poly metaphosphoric acid is \[{(HP{O_3})_n}\]. It has a linear polymer structure where the oxidation state of phosphorus is +5. The structure can be represented as:
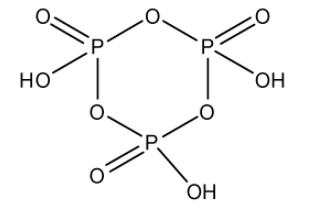
Cyclo trimetaphosphoric acid is a tribasic acid of phosphorus which has a formula \[{(HP{O_3})_3}\]. The three metaphosphoric acid molecules form a ring structure. Phosphorus is in the $ + 5$ oxidation state. In this structure, there are three \[P - OH\] bonds, three \[P = O\] bonds, and three \[P - O - P\] bonds:
Note: Important acidic nature of oxoacids of phosphorus: You are already aware of the term acids. These acids show varied physical and chemical properties of acids. Some simple ones are – they need a pH below 7, they turn blue paper red, they need a sour taste and that they react with alkalis to make salts. There is little difference of acidic strength among \[{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}{\text{, }}{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{{\text{3}}}}{\text{ and }}{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}\] because the hydrogen in these acids aren't all bonded to oxygen, and phosphorus isn't a highly electronegative element i.e. its oxides are less basic and electronegativities of ${\text{P}}$ and ${\text{H}}$ are almost the same. Hence, they all have almost similar acidic strength.

Complete Step by step solution:
Oxoacids of phosphorus which contain phosphorus-oxygen linkages are the most dominated subset in Phosphorus Chemistry.
Pyro phosphoric acid is a tetrabasic acid and prepared by heating orthophosphoric acid at about \[{250^o}{\text{C}}\] : \[2{H_3}P{O_4} \to {H_4}{P_2}{O_7} + {H_2}O\] The structure of Pyro phosphoric acid can be represented as:

The chemical formula of pyrophosphoric acid is \[{H_4}{P_2}{O_5}\]. The structure can be represented as:

The formula for Poly metaphosphoric acid is \[{(HP{O_3})_n}\]. It has a linear polymer structure where the oxidation state of phosphorus is +5. The structure can be represented as:

Cyclo trimetaphosphoric acid is a tribasic acid of phosphorus which has a formula \[{(HP{O_3})_3}\]. The three metaphosphoric acid molecules form a ring structure. Phosphorus is in the $ + 5$ oxidation state. In this structure, there are three \[P - OH\] bonds, three \[P = O\] bonds, and three \[P - O - P\] bonds:

Note: Important acidic nature of oxoacids of phosphorus: You are already aware of the term acids. These acids show varied physical and chemical properties of acids. Some simple ones are – they need a pH below 7, they turn blue paper red, they need a sour taste and that they react with alkalis to make salts. There is little difference of acidic strength among \[{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}{\text{, }}{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{{\text{3}}}}{\text{ and }}{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}\] because the hydrogen in these acids aren't all bonded to oxygen, and phosphorus isn't a highly electronegative element i.e. its oxides are less basic and electronegativities of ${\text{P}}$ and ${\text{H}}$ are almost the same. Hence, they all have almost similar acidic strength.

Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

The largest wind power cluster is located in the state class 11 social science CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

Which among the following are examples of coming together class 11 social science CBSE




