
Draw the structure of:
A.Pyrophosphoric acid
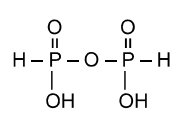
B.Pyro phosphorous acid
C.Poly metaphosphoric acid
D.Cyclo trimetaphosphoric acid
Answer
573.6k+ views
Hint: To answer this question, you should recall the concept of oxoacids of phosphorus. Phosphorus belongs to group 15 of the periodic table and forms various oxoacids: hypo phosphorous acid\[\left( {{H_3}P{O_2}} \right)\], Phosphorous acid\[\left( {{H_3}P{O_3}} \right)\], Hypo phosphoric acid\[\left( {{H_3}P{O_4}} \right)\], pyro phosphoric acid \[\left( {{H_4}{P_2}{O_7}} \right)\]and poly metaphosphoric acid\[{\left( {HP{O_3}} \right)_n}\].
Complete Step by step solution:
Oxoacids of phosphorus which contain phosphorus-oxygen linkages are the most dominated subset in Phosphorus Chemistry.
Pyro phosphoric acid is a tetrabasic acid and prepared by heating orthophosphoric acid at about \[{250^o}{\text{C}}\] : \[2{H_3}P{O_4} \to {H_4}{P_2}{O_7} + {H_2}O\] The structure of Pyro phosphoric acid can be represented as:
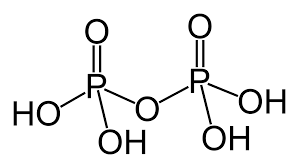
The chemical formula of pyrophosphoric acid is \[{H_4}{P_2}{O_5}\]. The structure can be represented as:
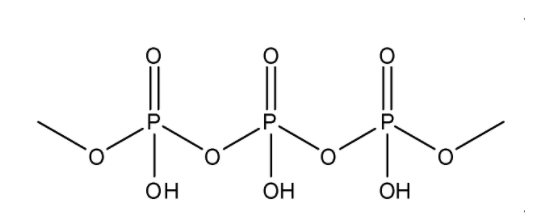
The formula for Poly metaphosphoric acid is \[{(HP{O_3})_n}\]. It has a linear polymer structure where the oxidation state of phosphorus is +5. The structure can be represented as:
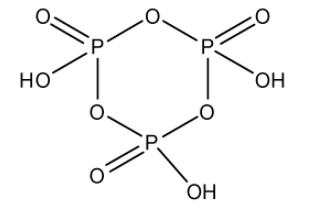
Cyclo trimetaphosphoric acid is a tribasic acid of phosphorus which has a formula \[{(HP{O_3})_3}\]. The three metaphosphoric acid molecules form a ring structure. Phosphorus is in the $ + 5$ oxidation state. In this structure, there are three \[P - OH\] bonds, three \[P = O\] bonds, and three \[P - O - P\] bonds:
Note: Important acidic nature of oxoacids of phosphorus: You are already aware of the term acids. These acids show varied physical and chemical properties of acids. Some simple ones are – they need a pH below 7, they turn blue paper red, they need a sour taste and that they react with alkalis to make salts. There is little difference of acidic strength among \[{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}{\text{, }}{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{{\text{3}}}}{\text{ and }}{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}\] because the hydrogen in these acids aren't all bonded to oxygen, and phosphorus isn't a highly electronegative element i.e. its oxides are less basic and electronegativities of ${\text{P}}$ and ${\text{H}}$ are almost the same. Hence, they all have almost similar acidic strength.

Complete Step by step solution:
Oxoacids of phosphorus which contain phosphorus-oxygen linkages are the most dominated subset in Phosphorus Chemistry.
Pyro phosphoric acid is a tetrabasic acid and prepared by heating orthophosphoric acid at about \[{250^o}{\text{C}}\] : \[2{H_3}P{O_4} \to {H_4}{P_2}{O_7} + {H_2}O\] The structure of Pyro phosphoric acid can be represented as:

The chemical formula of pyrophosphoric acid is \[{H_4}{P_2}{O_5}\]. The structure can be represented as:

The formula for Poly metaphosphoric acid is \[{(HP{O_3})_n}\]. It has a linear polymer structure where the oxidation state of phosphorus is +5. The structure can be represented as:

Cyclo trimetaphosphoric acid is a tribasic acid of phosphorus which has a formula \[{(HP{O_3})_3}\]. The three metaphosphoric acid molecules form a ring structure. Phosphorus is in the $ + 5$ oxidation state. In this structure, there are three \[P - OH\] bonds, three \[P = O\] bonds, and three \[P - O - P\] bonds:

Note: Important acidic nature of oxoacids of phosphorus: You are already aware of the term acids. These acids show varied physical and chemical properties of acids. Some simple ones are – they need a pH below 7, they turn blue paper red, they need a sour taste and that they react with alkalis to make salts. There is little difference of acidic strength among \[{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}{\text{, }}{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{{\text{3}}}}{\text{ and }}{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}\] because the hydrogen in these acids aren't all bonded to oxygen, and phosphorus isn't a highly electronegative element i.e. its oxides are less basic and electronegativities of ${\text{P}}$ and ${\text{H}}$ are almost the same. Hence, they all have almost similar acidic strength.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




