
How can I draw the Lewis structure for $Cl{{O}^{2-}}$ ?
Answer
565.5k+ views
Hint: As we know that Lewis structures are the diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that can exist in the molecule. It is found that these are also called electron dot structures. It basically adds up two lines between the atoms that shows shared pairs.
Complete Solution :
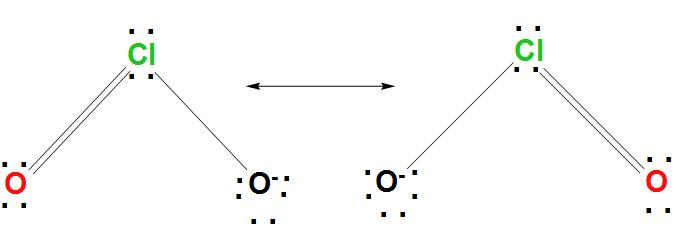
- In order to draw the Lewis structure for $Cl{{O}^{2-}}$, we can find the procedure here.
- As we know that for $Cl{{O}^{2-}}$, Cl is found to be the less electronegative atom, hence Cl is the central atom. The skeletal structure of Cl is found to be represented as:
O-Cl-O
- It is found that every atom has a formal charge. We can reduce the number of formal charges by moving a lone pair of electrons from oxygen to form a Cl=O bond.
- And we get two new structures in which one of the oxygen atoms has a formal charge equal to -1, and the other two atoms have a formal charge equal to zero.
- We can draw the Lewis structure for $Cl{{O}^{2-}}$as:
Note: - It is found that octet rule exists because the atoms of most of the elements become more stable by attaining the electronic configuration of an element. As we know that in octet rule atoms prefer to have eight electrons in the valence shell.
- Lewis structure mainly describes the structure of molecules with the help of symbols for the chemical species and also dot symbols.
Complete Solution :
- In order to draw the Lewis structure for $Cl{{O}^{2-}}$, we can find the procedure here.
- As we know that for $Cl{{O}^{2-}}$, Cl is found to be the less electronegative atom, hence Cl is the central atom. The skeletal structure of Cl is found to be represented as:
O-Cl-O
- It is found that every atom has a formal charge. We can reduce the number of formal charges by moving a lone pair of electrons from oxygen to form a Cl=O bond.
- And we get two new structures in which one of the oxygen atoms has a formal charge equal to -1, and the other two atoms have a formal charge equal to zero.
- We can draw the Lewis structure for $Cl{{O}^{2-}}$as:

Note: - It is found that octet rule exists because the atoms of most of the elements become more stable by attaining the electronic configuration of an element. As we know that in octet rule atoms prefer to have eight electrons in the valence shell.
- Lewis structure mainly describes the structure of molecules with the help of symbols for the chemical species and also dot symbols.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




