
How would you draw an alkane that has more than \[3\] but less than $10$ carbon atoms and only primary hydrogens?
Answer
553.8k+ views
Hint: The alkanes are a class of hydrocarbons which are saturated in nature, meaning, all the carbons are attached to each other through single bonds. These are hydrophobic in nature, and also non polar.
Complete step-by-step answer:In order to answer this question we must first understand what alkanes are. Alkanes are a group of hydrocarbons, in which carbons are attached to each other through single bonds and so, these are also called saturated hydrocarbons. The term primary hydrogen refers to the hydrogen which is attached to a carbon which has an attachment with only one other carbon. Now, in order to predict the structure of the compound which is asked, we need to understand that the structure could not be a single long hydrocarbon chain as the non-terminal carbons would be attached to two other carbons and so the criteria of primary hydrogen would not be fulfilled.
So, we need to think about a structure which has branching and also has more than three carbons.
Now, if we consider the structure where four methyl groups are attached to one carbon, then the carbon at the centre would not have any hydrogen, and the methyl groups would have three hydrogen each, making it primary hydrogens. The structure is shown below,
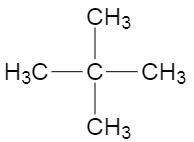
The name of this structure is \[2,2-\] dimethylpropane, and the total number of carbon atoms in the structure are five, which is more than three. Now, as we can see all the methyl groups are identical and are attached to only one other carbon which is the central carbon, which makes the hydrogen carried by the methyl groups, primary hydrogens.
So, the answer is \[2,2-\] dimethylpropane.
Note:The primary hydrogens are the type of hydrogen which are attached to the carbon which is attached to only one other carbon, and so the best structure which has all the primary hydrogens and has more than at least three carbons is \[2,2-\] dimethylpropane.
Complete step-by-step answer:In order to answer this question we must first understand what alkanes are. Alkanes are a group of hydrocarbons, in which carbons are attached to each other through single bonds and so, these are also called saturated hydrocarbons. The term primary hydrogen refers to the hydrogen which is attached to a carbon which has an attachment with only one other carbon. Now, in order to predict the structure of the compound which is asked, we need to understand that the structure could not be a single long hydrocarbon chain as the non-terminal carbons would be attached to two other carbons and so the criteria of primary hydrogen would not be fulfilled.
So, we need to think about a structure which has branching and also has more than three carbons.
Now, if we consider the structure where four methyl groups are attached to one carbon, then the carbon at the centre would not have any hydrogen, and the methyl groups would have three hydrogen each, making it primary hydrogens. The structure is shown below,

The name of this structure is \[2,2-\] dimethylpropane, and the total number of carbon atoms in the structure are five, which is more than three. Now, as we can see all the methyl groups are identical and are attached to only one other carbon which is the central carbon, which makes the hydrogen carried by the methyl groups, primary hydrogens.
So, the answer is \[2,2-\] dimethylpropane.
Note:The primary hydrogens are the type of hydrogen which are attached to the carbon which is attached to only one other carbon, and so the best structure which has all the primary hydrogens and has more than at least three carbons is \[2,2-\] dimethylpropane.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




